The Myxococcus xanthus spore cuticula protein C is a fragment of FibA, an extracellular metalloprotease produced exclusively in aggregated cells
- PMID: 22174937
- PMCID: PMC3236237
- DOI: 10.1371/journal.pone.0028968
The Myxococcus xanthus spore cuticula protein C is a fragment of FibA, an extracellular metalloprotease produced exclusively in aggregated cells
Abstract
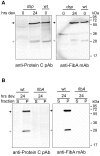
Myxococcus xanthus is a soil bacterium with a complex life cycle involving distinct cell fates, including production of environmentally resistant spores to withstand periods of nutrient limitation. Spores are surrounded by an apparently self-assembling cuticula containing at least Proteins S and C; the gene encoding Protein C is unknown. During analyses of cell heterogeneity in M. xanthus, we observed that Protein C accumulated exclusively in cells found in aggregates. Using mass spectrometry analysis of Protein C either isolated from spore cuticula or immunoprecipitated from aggregated cells, we demonstrate that Protein C is actually a proteolytic fragment of the previously identified but functionally elusive zinc metalloprotease, FibA. Subpopulation specific FibA accumulation is not due to transcriptional regulation suggesting post-transcriptional regulation mechanisms mediate its heterogeneous accumulation patterns.
Conflict of interest statement
Figures

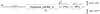

Similar articles
-
FibA and PilA act cooperatively during fruiting body formation of Myxococcus xanthus.Mol Microbiol. 2006 Sep;61(5):1283-93. doi: 10.1111/j.1365-2958.2006.05298.x. Mol Microbiol. 2006. PMID: 16925559
-
An extracellular matrix-associated zinc metalloprotease is required for dilauroyl phosphatidylethanolamine chemotactic excitation in Myxococcus xanthus.J Bacteriol. 2002 Mar;184(6):1678-84. doi: 10.1128/JB.184.6.1678-1684.2002. J Bacteriol. 2002. PMID: 11872719 Free PMC article.
-
The orphan response regulator DigR is required for synthesis of extracellular matrix fibrils in Myxococcus xanthus.J Bacteriol. 2006 Jun;188(12):4384-94. doi: 10.1128/JB.00189-06. J Bacteriol. 2006. PMID: 16740945 Free PMC article.
-
Highly Signal-Responsive Gene Regulatory Network Governing Myxococcus Development.Trends Genet. 2017 Jan;33(1):3-15. doi: 10.1016/j.tig.2016.10.006. Epub 2016 Dec 2. Trends Genet. 2017. PMID: 27916428 Free PMC article. Review.
-
Extracellular biology of Myxococcus xanthus.FEMS Microbiol Rev. 2010 Mar;34(2):89-106. doi: 10.1111/j.1574-6976.2009.00194.x. Epub 2009 Oct 20. FEMS Microbiol Rev. 2010. PMID: 19895646 Review.
Cited by
-
Spore formation in Myxococcus xanthus is tied to cytoskeleton functions and polysaccharide spore coat deposition.Mol Microbiol. 2012 Feb;83(3):486-505. doi: 10.1111/j.1365-2958.2011.07944.x. Epub 2011 Dec 21. Mol Microbiol. 2012. PMID: 22188356 Free PMC article.
-
A versatile class of cell surface directional motors gives rise to gliding motility and sporulation in Myxococcus xanthus.PLoS Biol. 2013 Dec;11(12):e1001728. doi: 10.1371/journal.pbio.1001728. Epub 2013 Dec 10. PLoS Biol. 2013. PMID: 24339744 Free PMC article.
-
Fatty acids from membrane lipids become incorporated into lipid bodies during Myxococcus xanthus differentiation.PLoS One. 2014 Jun 6;9(6):e99622. doi: 10.1371/journal.pone.0099622. eCollection 2014. PLoS One. 2014. PMID: 24906161 Free PMC article.
-
Myxococcus xanthus developmental cell fate production: heterogeneous accumulation of developmental regulatory proteins and reexamination of the role of MazF in developmental lysis.J Bacteriol. 2012 Jun;194(12):3058-68. doi: 10.1128/JB.06756-11. Epub 2012 Apr 6. J Bacteriol. 2012. PMID: 22493014 Free PMC article.
-
Myxobacteria: Moving, Killing, Feeding, and Surviving Together.Front Microbiol. 2016 May 26;7:781. doi: 10.3389/fmicb.2016.00781. eCollection 2016. Front Microbiol. 2016. PMID: 27303375 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

