Llama-derived single domain antibodies specific for Abrus agglutinin
- PMID: 22174977
- PMCID: PMC3237003
- DOI: 10.3390/toxins3111405
Llama-derived single domain antibodies specific for Abrus agglutinin
Abstract
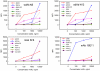
Llama derived single domain antibodies (sdAb), the recombinantly expressed variable heavy domains from the unique heavy-chain only antibodies of camelids, were isolated from a library derived from llamas immunized with a commercial abrin toxoid preparation. Abrin is a potent toxin similar to ricin in structure, sequence and mechanism of action. The selected sdAb were evaluated for their ability to bind to commercial abrin as well as abrax (a recombinant abrin A-chain), purified abrin fractions, Abrus agglutinin (a protein related to abrin but with lower toxicity), ricin, and unrelated proteins. Isolated sdAb were also evaluated for their ability to refold after heat denaturation and ability to be used in sandwich assays as both capture and reporter elements. The best binders were specific for the Abrus agglutinin, showing minimal binding to purified abrin fractions or unrelated proteins. These binders had sub nM affinities and regained most of their secondary structure after heating to 95 °C. They functioned well in sandwich assays. Through gel analysis and the behavior of anti-abrin monoclonal antibodies, we determined that the commercial toxoid preparation used for the original immunizations contained a high percentage of Abrus agglutinin, explaining the selection of Abrus agglutinin binders. Used in conjunction with anti-abrin monoclonal and polyclonal antibodies, these reagents can fill a role to discriminate between the highly toxic abrin and the related, but much less toxic, Abrus agglutinin and distinguish between different crude preparations.
Keywords: abrin; reversible refolding; single domain antibody.
Figures



Similar articles
-
Differentiation, Quantification and Identification of Abrin and Abrus precatorius Agglutinin.Toxins (Basel). 2021 Apr 18;13(4):284. doi: 10.3390/toxins13040284. Toxins (Basel). 2021. PMID: 33919561 Free PMC article.
-
A chimeric protein of abrin and Abrus precatorius agglutinin that neutralizes abrin mediated lethality in mice.Toxicon. 2017 Mar 1;127:122-129. doi: 10.1016/j.toxicon.2017.01.008. Epub 2017 Jan 11. Toxicon. 2017. PMID: 28088476
-
A Monoclonal-Monoclonal Antibody Based Capture ELISA for Abrin.Toxins (Basel). 2017 Oct 18;9(10):328. doi: 10.3390/toxins9100328. Toxins (Basel). 2017. PMID: 29057799 Free PMC article.
-
Gaps in forensic toxicological analysis: The veiled abrin.Toxicon. 2024 May 6;242:107684. doi: 10.1016/j.toxicon.2024.107684. Epub 2024 Mar 19. Toxicon. 2024. PMID: 38513827 Review.
-
Trends in the analysis of abrin poisoning for forensic purposes.J Forensic Leg Med. 2023 Aug;98:102564. doi: 10.1016/j.jflm.2023.102564. Epub 2023 Jul 13. J Forensic Leg Med. 2023. PMID: 37459705 Review.
Cited by
-
Isolation and epitope mapping of staphylococcal enterotoxin B single-domain antibodies.Sensors (Basel). 2014 Jun 19;14(6):10846-63. doi: 10.3390/s140610846. Sensors (Basel). 2014. PMID: 24949641 Free PMC article.
-
Antibody light chain variable domains and their biophysically improved versions for human immunotherapy.MAbs. 2014 Jan-Feb;6(1):219-35. doi: 10.4161/mabs.26844. MAbs. 2014. PMID: 24423624 Free PMC article.
-
Novel Phage Display-Derived Anti-Abrin Antibodies Confer Post-Exposure Protection against Abrin Intoxication.Toxins (Basel). 2018 Feb 13;10(2):80. doi: 10.3390/toxins10020080. Toxins (Basel). 2018. PMID: 29438273 Free PMC article.
-
Establishment and Comparison of Detection Methods for Ricin and Abrin Based on Their Depurination Activities.Toxins (Basel). 2025 Apr 3;17(4):177. doi: 10.3390/toxins17040177. Toxins (Basel). 2025. PMID: 40278675 Free PMC article.
-
Unraveling the Roots of Selectivity of Peptide Affinity Reagents for Structurally Similar Ribosomal Inactivating Protein Derivatives.Molecules. 2016 Nov 9;21(11):1504. doi: 10.3390/molecules21111504. Molecules. 2016. PMID: 27834872 Free PMC article.
References
-
- Endo Y., Mitsui K., Motizuki M., Tsurugi K. The mechanism of action of ricin and related toxic lectins on eukaryotic ribosomes-The site and characteristics of the modification in 28-S ribosoman-RNA caued by the toxins. J. Biol. Chem. 1987;262:5908–5912. - PubMed
-
- Kozlov Y.V., Sudarkina O.Y., Kurmanova A.G. Ribosome-inactivating lectins of plants. Mol. Biol. 2006;40:711–723. - PubMed
-
- Robertus J.D., Monzingo A.F. The structure of ribosome inactivating proteins. Mini-Rev. Med. Chem. 2004;4:477–486. - PubMed
-
- Wood K.A., Lord J.M., Wawrzynczak E.J., Piatak M. Preproabrin-Genomic cloning, characterization and the expression of the A-chain in Escheria-coli. Eur. J. Biochem. 1991;198:723–732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

