Antibodies against anthrax: mechanisms of action and clinical applications
- PMID: 22174979
- PMCID: PMC3237005
- DOI: 10.3390/toxins3111433
Antibodies against anthrax: mechanisms of action and clinical applications
Abstract
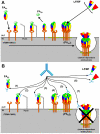
B. anthracis is a bioweapon of primary importance and its pathogenicity depends on its lethal and edema toxins, which belong to the A-B model of bacterial toxins, and on its capsule. These toxins are secreted early in the course of the anthrax disease and for this reason antibiotics must be administered early, in addition to other limitations. Antibodies (Abs) may however neutralize those toxins and target this capsule to improve anthrax treatment, and many Abs have been developed in that perspective. These Abs act at various steps of the cell intoxication and their mechanisms of action are detailed in the present review, presented in correlation with structural and functional data. The potential for clinical application is discussed for Abs targeting each step of entry, with four of these molecules already advancing to clinical trials. Paradoxically, certain Abs may also enhance the lethal toxin activity and this aspect will also be presented. The unique paradigm of Abs neutralizing anthrax toxins thus exemplifies how they may act to neutralize A-B toxins and, more generally, be active against infectious diseases.
Keywords: anthrax toxins; antibodies; edema factor; lethal factor; protective antigen.
Figures
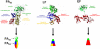
Similar articles
-
A Diverse Set of Single-domain Antibodies (VHHs) against the Anthrax Toxin Lethal and Edema Factors Provides a Basis for Construction of a Bispecific Agent That Protects against Anthrax Infection.J Biol Chem. 2016 Oct 7;291(41):21596-21606. doi: 10.1074/jbc.M116.749184. Epub 2016 Aug 18. J Biol Chem. 2016. PMID: 27539858 Free PMC article.
-
Revisiting the Concept of Targeting Only Bacillus anthracis Toxins as a Treatment for Anthrax.Antimicrob Agents Chemother. 2016 Jul 22;60(8):4878-85. doi: 10.1128/AAC.00546-16. Print 2016 Aug. Antimicrob Agents Chemother. 2016. PMID: 27270276 Free PMC article.
-
The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores.Microbiology (Reading). 2001 Jun;147(Pt 6):1677-1685. doi: 10.1099/00221287-147-6-1677. Microbiology (Reading). 2001. PMID: 11390699
-
Monoclonal antibody therapies against anthrax.Toxins (Basel). 2011 Aug;3(8):1004-19. doi: 10.3390/toxins3081004. Epub 2011 Aug 15. Toxins (Basel). 2011. PMID: 22069754 Free PMC article. Review.
-
Progress and novel strategies in vaccine development and treatment of anthrax.Immunol Rev. 2011 Jan;239(1):221-36. doi: 10.1111/j.1600-065X.2010.00969.x. Immunol Rev. 2011. PMID: 21198675 Review.
Cited by
-
Structural Basis for Binding of Neutralizing Antibodies to Clostridioides difficile Binary Toxin.J Bacteriol. 2023 Apr 25;205(4):e0045622. doi: 10.1128/jb.00456-22. Epub 2023 Mar 23. J Bacteriol. 2023. PMID: 36951574 Free PMC article.
-
Inhibition of Pore-Forming Proteins.Toxins (Basel). 2019 Sep 19;11(9):545. doi: 10.3390/toxins11090545. Toxins (Basel). 2019. PMID: 31546810 Free PMC article. Review.
-
Efficacy Projection of Obiltoxaximab for Treatment of Inhalational Anthrax across a Range of Disease Severity.Antimicrob Agents Chemother. 2016 Sep 23;60(10):5787-95. doi: 10.1128/AAC.00972-16. Print 2016 Oct. Antimicrob Agents Chemother. 2016. PMID: 27431222 Free PMC article.
-
Antibodies against Anthrax Toxins: A Long Way from Benchlab to the Bedside.Toxins (Basel). 2022 Feb 25;14(3):172. doi: 10.3390/toxins14030172. Toxins (Basel). 2022. PMID: 35324669 Free PMC article. Review.
-
Toxin-independent virulence of Bacillus anthracis in rabbits.PLoS One. 2014 Jan 8;9(1):e84947. doi: 10.1371/journal.pone.0084947. eCollection 2014. PLoS One. 2014. PMID: 24416317 Free PMC article.
References
-
- Inglesby T.V., O’Toole T., Henderson D.A., Bartlett J.G., Ascher M.S., Eitzen E., Friedlander A.M., Gerberding J., Hauer J., Hughes J., et al. Anthrax as a biological weapon, 2002: Updated recommendations for management. J. Am. Med. Assoc. 2002;287:2236–2252. - PubMed
-
- Friedlander A.M. Macrophages are sensitive to anthrax lethal toxin through an acid-dependent process. J. Biol. Chem. 1986;261:7123–7126. - PubMed
-
- Collier R.J., Young J.A. Anthrax toxin. Annu. Rev. Cell Dev. Biol. 2003;19:45–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

