The Trojan Horse Liposome Technology for Nonviral Gene Transfer across the Blood-Brain Barrier
- PMID: 22175028
- PMCID: PMC3228285
- DOI: 10.1155/2011/296151
The Trojan Horse Liposome Technology for Nonviral Gene Transfer across the Blood-Brain Barrier
Abstract
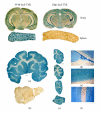
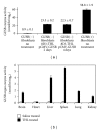
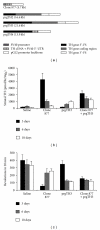
The application of blood-borne gene therapy protocols to the brain is limited by the presence of the blood-brain barrier (BBB). Viruses have been extensively used as gene delivery systems. However, their efficacy in brain is limited by the lack of transport across the BBB following intravenous (IV) administration. Recent progress in the "Trojan Horse Liposome" (THL) technology applied to transvascular non-viral gene therapy of the brain presents a promising solution to the trans-vascular brain gene delivery problem. THLs are comprised of immunoliposomes carrying nonviral gene expression plasmids. The tissue target specificity of the THL is provided by peptidomimetic monoclonal antibody (MAb) component of the THL, which binds to specific endogenous receptors located on both the BBB and on brain cellular membranes, for example, insulin receptor and transferrin receptor. These MAbs mediate (a) receptor-mediated transcytosis of the THL complex through the BBB, (b) endocytosis into brain cells and (c) transport to the brain cell nuclear compartment. The expression of the transgene in brain may be restricted using tissue/cell specific gene promoters. This manuscript presents an overview on the THL transport technology applied to brain disorders, including lysosomal storage disorders and Parkinson's disease.
Figures


References
-
- Pardridge WM. Drug and gene delivery to the brain: the vascular route. Neuron. 2002;36(4):555–558. - PubMed
-
- Mok KW, Lam AM, Cullis PR. Stabilized plasmid-lipid particles: factors influencing plasmid entrapment and transfection properties. Biochimica et Biophysica Acta. 1999;1419(2):137–150. - PubMed
-
- Boado RJ. Blood-brain barrier transport of non-viral gene and RNAi therapeutics. Pharmaceutical Research. 2007;24(9):1772–1787. - PubMed
-
- Byrnes AP, Rusby JE, Wood MJ, Charlton HM. Adenovirus gene transfer causes inflammation in the brain. Neuroscience. 1995;66(4):1015–1024. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

