Graphene multilayers as gates for multi-week sequential release of proteins from surfaces
- PMID: 22176729
- PMCID: PMC4040355
- DOI: 10.1021/nn202607r
Graphene multilayers as gates for multi-week sequential release of proteins from surfaces
Abstract
The ability to control the timing and order of release of different therapeutic drugs will play a pivotal role in improving patient care and simplifying treatment regimes in the clinic. The controlled sequential release of a broad range of small and macromolecules from thin film coatings offers a simple way to provide complex localized dosing in vivo. Here we show that it is possible to take advantage of the structure of certain nanomaterials to control release regimes from a scale of hours to months. Graphene oxide (GO) is a two-dimensional charged nanomaterial that can be used to create barrier layers in multilayer thin films, trapping molecules of interest for controlled release. Protein-loaded polyelectrolyte multilayer films were fabricated using layer-by-layer assembly incorporating a hydrolytically degradable cationic poly(β-amino ester) (Poly1) with a model protein antigen, ovalbumin (ova), in a bilayer architecture along with positively and negatively functionalized GO capping layers for the degradable protein films. Ova release without the GO layers takes place in less than 1 h but can be tuned to release from 30 to 90 days by varying the number of bilayers of functionalized GO in the multilayer architecture. We demonstrate that proteins can be released in sequence with multi-day gaps between the release of each species by incorporating GO layers between protein loaded layers. In vitro toxicity assays of the individual materials on proliferating hematopoietic stem cells (HSCs) indicated limited cytotoxic effects with HSCs able to survive for the full 10 days of normal culture in the presence of Poly1 and the GO sheets. This approach provides a new route for storage of therapeutics in a solid-state thin film for subsequent delivery in a time-controlled and sequential fashion.
© 2011 American Chemical Society
Figures


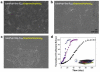
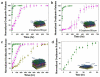


References
-
- Langer R, Folkman J. Polymers for Sustained-Release of Proteins and Other Macromolecules. Nature. 1976;263:797–800. - PubMed
-
- Pekarek KJ, Jacob JS, Mathiowitz E. Double-Walled Polymer Microspheres for Controlled Drug Release. Nature. 1991;367:258–260. - PubMed
-
- Langer R. Drug Delivery and Targeting. Nature. 1998;392:5–10. - PubMed
-
- Klugherz BD, Jones PL, Cui XM, Chen WL, Meneveau NF, DeFelice S, Connolly J, Wilensky RL, Levy RJ. Gene Delivery from a DNA Controlled-Release Stent in Porcine Coronary Arteries. Nat. Biotechnol. 2000;18:1181–1184. - PubMed
-
- Qiu Y, Park K. Environment-Sensitive Hydrogels for Drug Delivery. Adv. Drug Deliver. Rev. 2001;53:321–339. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

