Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor
- PMID: 22177419
- PMCID: PMC3334662
- DOI: 10.1186/ar3542
Decreased influenza-specific B cell responses in rheumatoid arthritis patients treated with anti-tumor necrosis factor
Abstract
Introduction: As a group, rheumatoid arthritis (RA) patients exhibit increased risk of infection, and those treated with anti-tumor necrosis factor (TNF) therapy are at further risk. This increased susceptibility may result from a compromised humoral immune response. Therefore, we asked if short-term effector (d5-d10) and memory (1 month or later) B cell responses to antigen were compromised in RA patients treated with anti-TNF therapy.
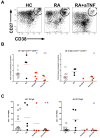
Methods: Peripheral blood samples were obtained from RA patients, including a subset treated with anti-TNF, and from healthy controls to examine influenza-specific responses following seasonal influenza vaccination. Serum antibody was measured by hemagglutination inhibition assay. The frequency of influenza vaccine-specific antibody secreting cells and memory B cells was measured by EliSpot. Plasmablast (CD19+IgD-CD27hiCD38hi) induction was measured by flow cytometry.
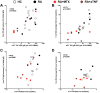
Results: Compared with healthy controls, RA patients treated with anti-TNF exhibited significantly decreased influenza-specific serum antibody and memory B cell responses throughout multiple years of the study. The short-term influenza-specific effector B cell response was also significantly decreased in RA patients treated with anti-TNF as compared with healthy controls, and correlated with decreased influenza-specific memory B cells and serum antibody present at one month following vaccination.
Conclusions: RA patients treated with anti-TNF exhibit a compromised immune response to influenza vaccine, consisting of impaired effector and consequently memory B cell and antibody responses. The results suggest that the increased incidence and severity of infection observed in this patient population could be a consequence of diminished antigen-responsiveness. Therefore, this patient population would likely benefit from repeat vaccination and from vaccines with enhanced immunogenicity.
Figures


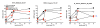
Comment in
-
Vaccination under TNF blockade - less effective, but worthwhile.Arthritis Res Ther. 2012 May 14;14(3):117. doi: 10.1186/ar3808. Arthritis Res Ther. 2012. PMID: 22577892 Free PMC article.
References
-
- van Assen S, Elkayam O, Agmon-Levin N, Cervera R, Doran MF, Dougados M, Emery P, Geborek P, Ioannidis JP, Jayne DR, Kallenberg CG, Müller-Ladner U, Shoenfeld Y, Stojanovich L, Valesini G, Wulffraat NM, Bijl M. Vaccination in adult patients with auto-immune inflammatory rheumatic diseases: a systematic literature review for the European League Against Rheumatism evidence-based recommendations for vaccination in adult patients with auto-immune inflammatory rheumatic diseases. Autoimmun Rev. 2011;10:341–352. doi: 10.1016/j.autrev.2010.12.003. - DOI - PubMed
-
- Gelinck LB, van der Bijl AE, Beyer WE, Visser LG, Huizinga TW, van Hogezand RA, Rimmelzwaan GF, Kroon FP. The effect of anti-tumour necrosis factor alpha treatment on the antibody response to influenza vaccination. Ann Rheum Dis. 2008;67:713–716. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

