Whole blood flow cytometry measurements of in vivo platelet activation in critically-Ill patients are influenced by variability in blood sampling techniques
- PMID: 22178064
- PMCID: PMC3323728
- DOI: 10.1016/j.thromres.2011.11.031
Whole blood flow cytometry measurements of in vivo platelet activation in critically-Ill patients are influenced by variability in blood sampling techniques
Abstract
Introduction: Flow cytometry is often used to measure in vivo platelet activation in critically-ill patients. Variability in blood sampling techniques, which may confound these measurements, remains poorly characterized.
Materials and methods: Platelet activation was measured by flow cytometry performed on arterial and venous blood from 116 critically-ill patients. We determined how variability in vascular sampling site, processing times, and platelet counts influenced levels of platelet-monocyte aggregates (PMA), PAC-1 binding (for glycoprotein (GP) IIbIIIa), and P-selectin (P-SEL) expression.
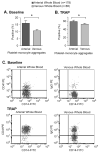
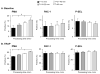
Results: Levels of PMA, but not PAC-1 binding or P-SEL expression, were significantly affected by variability in vascular sampling site. Average PMA levels were approximately 60% higher in whole blood drawn from an arterial vessel compared to venous blood (16.2±1.8% vs. 10.7±1.2%, p<0.05). Levels of PMA in both arterial and venous blood increased significantly during ex vivo processing delays (1.7% increase for every 10 minute delay, p<0.05). In contrast, PAC-1 binding and P-SEL expression were unaffected by processing delays. Levels of PMA, but not PAC-1 binding or P-SEL expression, were correlated with platelet count quartiles (9.4±1.6% for the lowest quartile versus 15.4±1.6% for the highest quartile, p<0.05).
Conclusions: In critically-ill patients, variability in vascular sampling site, processing times, and platelet counts influence levels of PMA, but not PAC-1 binding or P-SEL expression. These data demonstrate the need for rigorous adherence to blood sampling protocols, particularly when levels of PMA, which are most sensitive to variations in blood collection, are measured for detection of in vivo platelet activation.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
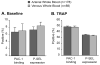
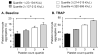
Similar articles
-
The importance of sampling site in the measurement of whole-blood platelet flow cytometry.J Cardiothorac Vasc Anesth. 1998 Jun;12(3):309-13. doi: 10.1016/s1053-0770(98)90012-x. J Cardiothorac Vasc Anesth. 1998. PMID: 9636914
-
The effect of red blood cell transfusion on platelet function in critically ill patients.Thromb Res. 2019 Dec;184:115-121. doi: 10.1016/j.thromres.2019.10.028. Epub 2019 Nov 1. Thromb Res. 2019. PMID: 31731068
-
[Whole blood flow cytometry for detection of activated platelets. I. Platelet identification, blood collection and storage].Rinsho Byori. 2001 Apr;49(4):402-7. Rinsho Byori. 2001. PMID: 11391956 Japanese.
-
Platelet Function Determined by Flow Cytometry: New Perspectives?Semin Thromb Hemost. 2016 Apr;42(3):268-81. doi: 10.1055/s-0035-1570082. Epub 2016 Feb 17. Semin Thromb Hemost. 2016. PMID: 26886398 Review.
-
Platelets as predictors of vascular risk: is there a practical index of platelet activity?Clin Appl Thromb Hemost. 2003 Jul;9(3):177-90. doi: 10.1177/107602960300900301. Clin Appl Thromb Hemost. 2003. PMID: 14507105 Review.
Cited by
-
Emerging evidence for platelets as immune and inflammatory effector cells.Front Immunol. 2014 Dec 18;5:653. doi: 10.3389/fimmu.2014.00653. eCollection 2014. Front Immunol. 2014. PMID: 25566264 Free PMC article. Review.
-
Platelet activation increases in patients undergoing vascular surgery.Thromb Res. 2014 Nov;134(5):952-6. doi: 10.1016/j.thromres.2014.08.009. Epub 2014 Aug 23. Thromb Res. 2014. PMID: 25208456 Free PMC article.
-
Impact of sampling technique, anticoagulant, processing delay, and temperature on murine platelet function in whole blood.Res Pract Thromb Haemost. 2025 May 8;9(4):102883. doi: 10.1016/j.rpth.2025.102883. eCollection 2025 May. Res Pract Thromb Haemost. 2025. PMID: 40519880 Free PMC article.
-
Cytokine signatures in chronic fatigue syndrome patients: a Case Control Study and the effect of anakinra treatment.J Transl Med. 2017 Dec 29;15(1):267. doi: 10.1186/s12967-017-1371-9. J Transl Med. 2017. PMID: 29284500 Free PMC article. Clinical Trial.
-
Granzyme A in Human Platelets Regulates the Synthesis of Proinflammatory Cytokines by Monocytes in Aging.J Immunol. 2018 Jan 1;200(1):295-304. doi: 10.4049/jimmunol.1700885. Epub 2017 Nov 22. J Immunol. 2018. PMID: 29167233 Free PMC article.
References
-
- Schwertz H, Weyrich AS, Zimmerman GA. Cellular interactions of platelets, leukocytes and endothelium in systemic inflammatory responses and sepsis. In: Castro Faria Neto H, Marcus D, editors. Sepsis: From Bench to Bedside. Rio de Janerio: Revinter Press; 2007. pp. 107–120.
-
- Gawaz M, Dickfeld T, Bogner C, Fateh-Moghadam S, Neumann FJ. Platelet function in septic multiple organ dysfunction syndrome. Intensive care medicine. 1997;23(4):379–385. - PubMed
-
- Gawaz M, Fateh-Moghadam S, Pilz G, Gurland HJ, Werdan K. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest. 1995;25(11):843–851. - PubMed
-
- Michelson AD, Furman MI. Laboratory markers of platelet activation and their clinical significance. Curr Opin Hematol. 1999;6(5):342–348. - PubMed
-
- Michelson AD. Flow cytometry: a clinical test of platelet function. Blood. 1996;87 (12):4925–4936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

