mTOR activates hypoxia-inducible factor-1α and inhibits neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia
- PMID: 22178140
- PMCID: PMC3525671
- DOI: 10.1016/j.neulet.2011.11.058
mTOR activates hypoxia-inducible factor-1α and inhibits neuronal apoptosis in the developing rat brain during the early phase after hypoxia-ischemia
Abstract
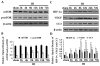
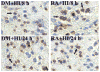
The mammalian target of rapamycin (mTOR) exerts neuroprotective effects under hypoxic or ischemic conditions. To explore whether mTOR participates in neuroprotective signaling through regulation of hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor (VEGF) and neuronal apoptosis in developing rat brain with hypoxia-ischemia (HI), we operated on postnatal day 10 rats by ligating the common carotid artery followed by exposure to systemic hypoxia. Brains were collected at various intervals to detect the expression of mTOR, phosphorylated mTOR (p-mTOR), HIF-1α, VEGF and cleaved caspase 3 (CC3), using immunohistochemistry and Western blot analysis. We also used terminal deoxynucleotidyl transferase-mediated dUTP-nick end labeling (TUNEL) to detect neuronal apoptosis. The p-mTOR protein expression increased at 2h after HI, peaked at 8h, lasted 24h, and then dropped to the basal level. Also, the expression of HIF-1α and VEGF was significantly enhanced and peaked at 8h after HI. Up-regulated expression of CC3 was observed at 2h, peaked at 24h, and lasted 72h after HI. Increased neuronal apoptosis is associated with reduced HIF-1α and VEGF expression. Furthermore, pretreatment with rapamycin, a mTOR specific inhibitor, significantly inhibited HIF-1α and VEGF protein after HI. The expression of CC3 and the number of TUNEL-positive cells were up-regulated at 8h and down-regulated at 24h after HI in the rapamycin-treated group. Our findings suggest that mTOR may participate in the regulation of HIF-1α, VEGF and neuronal apoptosis, serving neuroprotective functions after HI in developing rat brain.
Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
All of the authors involved in preparation of the above manuscript declare no conflict of interest in any form.
Figures


References
-
- Balduini W, Carloni S, Buonocore G. Autophagy in hypoxia-ischemia induced brain injury: evidence and speculations. Autophagy. 2009;5(2):221–223. - PubMed
-
- Blomgren K, Leist M, Groc L. Pathological apoptosis in the developing brain. Apoptosis. 2007;12(5):993–1010. - PubMed
-
- Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32(3):329–339. - PubMed
-
- Del Bufalo D, Ciuffreda L, Trisciuoglio D, Desideri M, Cognetti F, Zupi G, Milella M. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66(11):5549–5554. - PubMed
-
- Fahling M. Cellular oxygen sensing, signalling and how to survive translational arrest in hypoxia. Acta Physiol (Oxf) 2009;195(2):205–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

