In vitro enrofloxacin binding in human fecal slurries
- PMID: 22178170
- PMCID: PMC12415927
- DOI: 10.1016/j.yrtph.2011.11.013
In vitro enrofloxacin binding in human fecal slurries
Abstract
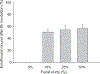
Most antibiotic inactivation studies have been conducted through in vitro incubations of human use aminoglycosides, beta-lactams, and fluoroquinolones, usually at fecal concentrations expected with therapeutic dose regimens in humans and animals. Less is known about the inactivation of these molecules when ingested at concentrations consistent with residue levels present in animal-derived foods from antibiotic treated animals. In this investigation, we used the fluoroquinolone, enrofloxacin which is specifically marketed for veterinary medicine as test compound. Fecal suspensions at 10%, 25%, and 50% (w/v) were subjected to physicochemical and molecular characterization and used in the drug binding studies. The fecal binding of enrofloxacin added at concentrations of 0.06, 0.1, 1, 5, 15, 50, and 150 mg/L was determined in various fecal slurry suspensions using analytical chemistry and microbiological assay methods. There was consistent correlation between both assay methods. By the analytical chemistry assay, the 10%, 25% and 50% diluted autoclaved fecal samples dosed with enrofloxacin showed binding of 50±4.6%, 54±6.5% and 56±6.8% of the enrofloxacin, respectively. Binding of enrofloxacin to fecal contents occurred rapidly within 10 min and remained constant over the incubation period. Denaturing gradient gel electrophoreses and pyrosequencing analysis showed varied profiles of the bacterial composition of the human intestinal microbiota for fecal samples from different individuals. This study provided information on methodological questions that have concerned regulatory authorities on in vitro testing to determine if concentrations of veterinary antimicrobial agent residues entering the human colon remain microbiologically active.
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures



References
-
- Adjei MD, Heinze TM, Deck J, Freeman JP, Williams AJ, Sutherland JB, 2007. Acetylation and nitrosation of ciprofloxacin by environmental strains of Mycobacteria. Can. J. Microbiol. 53, 144–147. - PubMed
-
- Ahn YB, Häggblom MM, Fennell DE, 2005. Co-amendment with halogenated compounds enhances anaerobic microbial dechlorination of 1,2,3,4-tetrachlorodibenzo-p-dioxin and 1,2,3,4-tetrachlorodibenzofuran in estuarine sediments. Environ. Toxicol. Chem. 24, 2775–2784. - PubMed
-
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI, 2005. Host-bacterial mutualism in the human intestine. Science 307, 1915–1920. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

