Cryo X-ray nano-tomography of vaccinia virus infected cells
- PMID: 22178221
- PMCID: PMC7119024
- DOI: 10.1016/j.jsb.2011.12.001
Cryo X-ray nano-tomography of vaccinia virus infected cells
Abstract
We have performed full-field cryo X-ray microscopy in the water window photon energy range on vaccinia virus (VACV) infected cells to produce tomographic reconstructions. PtK2 cells were infected with a GFP-expressing VACV strain and frozen by plunge fast freezing. The infected cells were selected by light fluorescence microscopy of the GFP marker and subsequently imaged in the X-ray microscope under cryogenic conditions. Tomographic tilt series of X-ray images were used to yield three-dimensional reconstructions showing different cell organelles (nuclei, mitochondria, filaments), together with other structures derived from the virus infection. Among them, it was possible to detect viral factories and two types of viral particles related to different maturation steps of VACV (immature and mature particles), which were compared to images obtained by standard electron microscopy of the same type of cells. In addition, the effect of radiation damage during X-ray tomographic acquisition was analyzed. Thin sections studied by electron microscopy revealed that the morphological features of the cells do not present noticeable changes after irradiation. Our findings show that cryo X-ray nano-tomography is a powerful tool for collecting three-dimensional structural information from frozen, unfixed, unstained whole cells with sufficient resolution to detect different virus particles exhibiting distinct maturation levels.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



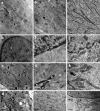
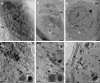


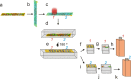
References
-
- Agulleiro J.I., Fernandez J.J. Fast tomographic reconstruction on multicore computers. Bioinformatics. 2011;27:582–583. - PubMed
-
- Baumeister W., Steven A.C. Macromolecular electron microscopy in the era of structural genomics. Trends Biochem. Sci. 2000;25:624–631. - PubMed
-
- Carrascosa J.L., Chichon F.J., Pereiro E., Rodriguez M.J., Fernandez J.J., Esteban M., Heim S., Guttmann P., Schneider G. Cryo-X-ray tomography of vaccinia virus membranes and inner compartments. J. Struct. Biol. 2009;168:234–239. - PubMed
-
- Condit R.C., Moussatche N., Traktman P. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 2006;66:31–124. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

