Radical reactions of thiamin pyrophosphate in 2-oxoacid oxidoreductases
- PMID: 22178227
- PMCID: PMC3438339
- DOI: 10.1016/j.bbapap.2011.11.010
Radical reactions of thiamin pyrophosphate in 2-oxoacid oxidoreductases
Abstract
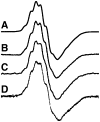
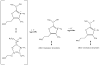
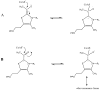
Thiamin pyrophosphate (TPP) is essential in carbohydrate metabolism in all forms of life. TPP-dependent decarboxylation reactions of 2-oxo-acid substrates result in enamine adducts between the thiazolium moiety of the coenzyme and decarboxylated substrate. These central enamine intermediates experience different fates from protonation in pyruvate decarboxylase to oxidation by the 2-oxoacid dehydrogenase complexes, the pyruvate oxidases, and 2-oxoacid oxidoreductases. Virtually all of the TPP-dependent enzymes, including pyruvate decarboxylase, can be assayed by 1-electron redox reactions linked to ferricyanide. Oxidation of the enamines is thought to occur via a 2-electron process in the 2-oxoacid dehydrogenase complexes, wherein acyl group transfer is associated with reduction of the disulfide of the lipoamide moiety. However, discrete 1-electron steps occur in the oxidoreductases, where one or more [4Fe-4S] clusters mediate the electron transfer reactions to external electron acceptors. These radical intermediates can be detected in the absence of the acyl-group acceptor, coenzyme A (CoASH). The π-electron system of the thiazolium ring stabilizes the radical. The extensively delocalized character of the radical is evidenced by quantitative analysis of nuclear hyperfine splitting tensors as detected by electron paramagnetic resonance (EPR) spectroscopy and by electronic structure calculations. The second electron transfer step is markedly accelerated by the presence of CoASH. While details of the second electron transfer step and its facilitation by CoASH remain elusive, expected redox properties of potential intermediates limit possible scenarios. This article is part of a Special Issue entitled: Radical SAM enzymes and Radical Enzymology.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures




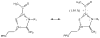
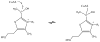

Similar articles
-
Mechanism of the Clostridium thermoaceticum pyruvate:ferredoxin oxidoreductase: evidence for the common catalytic intermediacy of the hydroxyethylthiamine pyropyrosphate radical.Biochemistry. 1997 Jul 15;36(28):8484-94. doi: 10.1021/bi970403k. Biochemistry. 1997. PMID: 9214293
-
Pulsed electron paramagnetic resonance experiments identify the paramagnetic intermediates in the pyruvate ferredoxin oxidoreductase catalytic cycle.J Am Chem Soc. 2006 Mar 29;128(12):3888-9. doi: 10.1021/ja0585275. J Am Chem Soc. 2006. PMID: 16551078 Free PMC article.
-
EPR spectroscopic and computational characterization of the hydroxyethylidene-thiamine pyrophosphate radical intermediate of pyruvate:ferredoxin oxidoreductase.Biochemistry. 2006 Jun 13;45(23):7122-31. doi: 10.1021/bi0602516. Biochemistry. 2006. PMID: 16752902 Free PMC article.
-
Reaction mechanisms of thiamin diphosphate enzymes: redox reactions.FEBS J. 2009 May;276(9):2454-68. doi: 10.1111/j.1742-4658.2009.06966.x. Epub 2009 Mar 16. FEBS J. 2009. PMID: 19476487 Review.
-
Identification and function of auxiliary iron-sulfur clusters in radical SAM enzymes.Biochim Biophys Acta. 2012 Nov;1824(11):1196-212. doi: 10.1016/j.bbapap.2012.07.009. Epub 2012 Jul 28. Biochim Biophys Acta. 2012. PMID: 22846545 Review.
Cited by
-
One-carbon chemistry of oxalate oxidoreductase captured by X-ray crystallography.Proc Natl Acad Sci U S A. 2016 Jan 12;113(2):320-5. doi: 10.1073/pnas.1518537113. Epub 2015 Dec 28. Proc Natl Acad Sci U S A. 2016. PMID: 26712008 Free PMC article.
-
Formation of reactive oxygen species by human and bacterial pyruvate and 2-oxoglutarate dehydrogenase multienzyme complexes reconstituted from recombinant components.Free Radic Biol Med. 2015 Dec;89:642-50. doi: 10.1016/j.freeradbiomed.2015.10.001. Epub 2015 Oct 9. Free Radic Biol Med. 2015. PMID: 26456061 Free PMC article.
-
The redox landscape of pyruvate:ferredoxin oxidoreductases reveals often conserved Fe-S cluster potentials.J Biol Chem. 2025 Jun 16;301(8):110380. doi: 10.1016/j.jbc.2025.110380. Online ahead of print. J Biol Chem. 2025. PMID: 40533063 Free PMC article.
-
Crystal structures of archaeal 2-oxoacid:ferredoxin oxidoreductases from Sulfolobus tokodaii.Sci Rep. 2016 Sep 13;6:33061. doi: 10.1038/srep33061. Sci Rep. 2016. PMID: 27619895 Free PMC article.
-
Structural organization of pyruvate: ferredoxin oxidoreductase from the methanogenic archaeon Methanosarcina acetivorans.Structure. 2024 Nov 7;32(11):1963-1972.e3. doi: 10.1016/j.str.2024.08.011. Epub 2024 Sep 11. Structure. 2024. PMID: 39265575 Free PMC article.
References
-
- Frey PA, Hegeman AD. Enzymatic reaction mechanisms. Oxford University Press; New York: 2007.
-
- Breslow R. On the mechanism of thiamine action. IV. Evidence from studies on model systems. J Am Chem Soc. 1958;80:3719–3726.
-
- Krampitz LO, Votaw R. α-Hydroxyethylthiamine diphosphate and α, β-dihydroxyethylthiamine disphosphate. Method Enzymol. 1966;9:65–68.
-
- Frey PA. Chemical intermediates in catalysis by thiamine diphosphate. In: Jordan J, Patel M, Jordan F, editors. Thiamine: Catalytic Mechanisms in Normal and Disease States. Marcel Dekker; New York: 2003. pp. 1–13.
-
- Hager LP. Ph D dissertation. University of Illinois; 1953. The enzymatic steps in α-keto acid oxidations.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

