Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment
- PMID: 22178238
- PMCID: PMC3402174
- DOI: 10.1016/j.yexcr.2011.11.014
Lactate is a mediator of metabolic cooperation between stromal carcinoma associated fibroblasts and glycolytic tumor cells in the tumor microenvironment
Abstract
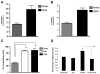
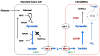
Human mesenchymal stem cells (hMSCs) are bone marrow-derived stromal cells, which play a role in tumor progression. We have shown earlier that breast cancer cells secrete higher levels of interleukin-6 (IL-6) under hypoxia, leading to the recruitment of hMSCs towards hypoxic tumor cells. We found that (i) MDA-MB-231 cells secrete significantly higher levels of lactate (3-fold more) under hypoxia (1% O(2)) than under 20% O(2) and (ii) lactate recruits hMSCs towards tumor cells by activating signaling pathways to enhance migration. The mRNA and protein expression of functional MCT1 in hMSCs is increased in response to lactate exposure. Thus, we hypothesized that hMSCs and stromal carcinoma associated fibroblasts (CAFs) in the tumor microenvironment have the capacity to take up lactate expelled from tumor cells and use it as a source of energy. Our (13)C NMR spectroscopic measurements indicate that (13)C-lactate is converted to (13)C-alpha ketoglutarate in hMSCs and CAFs supporting this hypothesis. To our knowledge this is the first in vitro model system demonstrating that hMSCs and CAFs can utilize lactate produced by tumor cells.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures

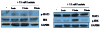

References
-
- Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13:472–482. - PubMed
-
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. - PubMed
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. - PubMed
-
- Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

