Dysfunction of nodes of Ranvier: a mechanism for anti-ganglioside antibody-mediated neuropathies
- PMID: 22178332
- PMCID: PMC3268874
- DOI: 10.1016/j.expneurol.2011.11.039
Dysfunction of nodes of Ranvier: a mechanism for anti-ganglioside antibody-mediated neuropathies
Abstract
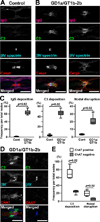
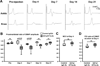
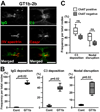
Autoantibodies against gangliosides GM1 or GD1a are associated with acute motor axonal neuropathy (AMAN) and acute motor-sensory axonal neuropathy (AMSAN), whereas antibodies to GD1b ganglioside are detected in acute sensory ataxic neuropathy (ASAN). These neuropathies have been proposed to be closely related and comprise a continuous spectrum, although the underlying mechanisms, especially for sensory nerve involvement, are still unclear. Antibodies to GM1 and GD1a have been proposed to disrupt the nodes of Ranvier in motor nerves via complement pathway. We hypothesized that the disruption of nodes of Ranvier is a common mechanism whereby various anti-ganglioside antibodies found in these neuropathies lead to nervous system dysfunction. Here, we show that the IgG monoclonal anti-GD1a/GT1b antibody injected into rat sciatic nerves caused deposition of IgG and complement products on the nodal axolemma and disrupted clusters of nodal and paranodal molecules predominantly in motor nerves, and induced early reversible motor nerve conduction block. Injection of IgG monoclonal anti-GD1b antibody induced nodal disruption predominantly in sensory nerves. In an ASAN rabbit model associated with IgG anti-GD1b antibodies, complement-mediated nodal disruption was observed predominantly in sensory nerves. In an AMAN rabbit model associated with IgG anti-GM1 antibodies, complement attack of nodes was found primarily in motor nerves, but occasionally in sensory nerves as well. Periaxonal macrophages and axonal degeneration were observed in dorsal roots from ASAN rabbits and AMAN rabbits. Thus, nodal disruption may be a common mechanism in immune-mediated neuropathies associated with autoantibodies to gangliosides GM1, GD1a, or GD1b, providing an explanation for the continuous spectrum of AMAN, AMSAN, and ASAN.
Copyright © 2011 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures





Comment in
-
Progress in untying the Gordian nodes of Ranvier in Guillain-Barré Syndrome.Exp Neurol. 2012 May;235(1):211-3. doi: 10.1016/j.expneurol.2012.03.001. Epub 2012 Mar 17. Exp Neurol. 2012. PMID: 22449474 No abstract available.
Similar articles
-
Anti-ganglioside antibodies can bind peripheral nerve nodes of Ranvier and activate the complement cascade without inducing acute conduction block in vitro.Brain. 1999 May;122 ( Pt 5):807-16. doi: 10.1093/brain/122.5.807. Brain. 1999. PMID: 10355667
-
Gangliosides contribute to stability of paranodal junctions and ion channel clusters in myelinated nerve fibers.Glia. 2007 May;55(7):746-57. doi: 10.1002/glia.20503. Glia. 2007. PMID: 17352383
-
Association of antibodies to ganglioside complexes and conduction blocks in axonal Guillain-Barré syndrome presenting as acute motor conduction block neuropathy.J Peripher Nerv Syst. 2014 Jun;19(2):115-20. doi: 10.1111/jns5.12060. J Peripher Nerv Syst. 2014. PMID: 24750296
-
A common mechanism and a new categorization for anti-ganglioside antibody-mediated neuropathies.Exp Neurol. 2012 Jun;235(2):513-6. doi: 10.1016/j.expneurol.2012.03.023. Epub 2012 Apr 6. Exp Neurol. 2012. PMID: 22507308 Review.
-
Natura non facit saltus in anti-ganglioside antibody-mediated neuropathies.Muscle Nerve. 2013 Oct;48(4):484-7. doi: 10.1002/mus.23881. Muscle Nerve. 2013. PMID: 23625341 Review.
Cited by
-
Rapid and reversible responses to IVIG in autoimmune neuromuscular diseases suggest mechanisms of action involving competition with functionally important autoantibodies.J Peripher Nerv Syst. 2013 Dec;18(4):275-96. doi: 10.1111/jns5.12048. J Peripher Nerv Syst. 2013. PMID: 24200120 Free PMC article. Review.
-
Pathology of Initial Axon Segments in Chronic Inflammatory Demyelinating Polyradiculoneuropathy and Related Disorders.Int J Mol Sci. 2022 Nov 7;23(21):13621. doi: 10.3390/ijms232113621. Int J Mol Sci. 2022. PMID: 36362407 Free PMC article. Review.
-
Anti-CNTN1 IgG3 induces acute conduction block and motor deficits in a passive transfer rat model.J Neuroinflammation. 2019 Apr 5;16(1):73. doi: 10.1186/s12974-019-1462-z. J Neuroinflammation. 2019. PMID: 30953561 Free PMC article.
-
Serum Antibodies to Glycans in Peripheral Neuropathies.Mol Neurobiol. 2017 Mar;54(2):1564-1567. doi: 10.1007/s12035-016-9775-8. Epub 2016 Feb 11. Mol Neurobiol. 2017. PMID: 26867654 Review.
-
Molecular, Electrophysiological, and Ultrasonographic Differences in Selected Immune-Mediated Neuropathies with Therapeutic Implications.Int J Mol Sci. 2023 May 24;24(11):9180. doi: 10.3390/ijms24119180. Int J Mol Sci. 2023. PMID: 37298132 Free PMC article. Review.
References
-
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. βIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J. Cell Biol. 2000;151:985–1001. - PMC - PubMed
-
- Bergman E, Fundin BT, Ulfhake B. Effects of aging and axotomy on the expression of neurotrophin receptors in primary sensory neurons. J. Comp. Neurol. 1999;410:368–386. - PubMed
-
- Capasso M, Notturno F, Manzoli C, Uncini A. Involvement of sensory fibres in axonal subtypes of Guillain-Barré syndrome. J. Neurol. Neurosurg. Psychiatry. 2011;82:664–670. - PubMed
-
- Castro J, Negredo P, Avendaño C. Fiber composition of the rat sciatic nerve and its modification during regeneration through a sieve electrode. Brain Res. 2008;1190:65–77. - PubMed
-
- Comín R, Yuki N, Lopez PH, Nores GA. High affinity of anti-GM1 antibodies is associated with disease onset in experimental neuropathy. J. Neurosci. Res. 2006;84:1085–1090. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

