Combination effects of cigarette smoke extract and ambient ultrafine particles on endothelial cells
- PMID: 22178768
- PMCID: PMC3273600
- DOI: 10.1016/j.tiv.2011.12.001
Combination effects of cigarette smoke extract and ambient ultrafine particles on endothelial cells
Abstract
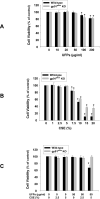
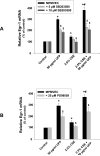
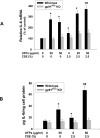
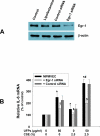
Previous studies have shown that ambient ultrafine particles with diameters less than 100nm (UFPs) can pass from the lungs to the circulation because of their very small diameter, and induce lung oxidative stress with a resultant dysfunction of lung endothelial cells. However, no studies have addressed the potential combined effects of UFPs and cigarette smoke on vascular endothelial cells. We hypothesized that co-exposure to UFPs and cigarette smoke extract (CSE) may cause combined effects on activation of endothelial cells and dysfunction of endothelium by oxidative stress through activation of NADPH oxidase. We determined the effects of UFPs with or without CSE on mouse pulmonary microvascular endothelial cells (MPMVEC) obtained from C57BL/6J (wild-type) and gp91(phox) knock-out mice (gp91(phox) is one of the key components of NADPH oxidase, one of ROS generators). Our results showed that exposure of MPMVEC from wild-type mice to UFPs or CSE, at a non-toxic dose, induced reactive oxygen species (ROS) generation, increased phosphorylation of p38 and Erk1/2, and up-regulated early growth response -1 (Egr-1) and IL-6 genes. These effects were significantly enhanced when cells were co-exposed to both UFPs and CSE. However, exposure of MPMVEC from gp91(phox) knock-out mice did not induce the above effects. Furthermore, UFPs- and/or CSE-induced Egr-1 mRNA upregulation was attenuated significantly when cells were pre-treated with p38 specific inhibitor, SB 203580, or MEK1/2 inhibitor, PD98059, and Egr-1 siRNA treatment abolished UFPs- and/or CSE-induced overexpression of IL-6. Our results suggest that UFPs and/or CSE caused activation of NADPH oxidase, resulting in ROS generation that led to activation of MAPKs through induced phosphorylation of p38 and ERK1/2 MAPKs and upregulation of Egr-1. Those effects may further result in endothelial dysfunction through production of cytokines such as IL-6. Our results suggest that co-exposure to UFPs and CSE causes enhanced injury to endothelial cells.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures





Similar articles
-
Activation of endothelial cells after exposure to ambient ultrafine particles: the role of NADPH oxidase.Toxicol Appl Pharmacol. 2009 Apr 15;236(2):183-93. doi: 10.1016/j.taap.2009.01.017. Epub 2009 Feb 5. Toxicol Appl Pharmacol. 2009. PMID: 19371610
-
Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice.Am J Pathol. 2008 May;172(5):1222-37. doi: 10.2353/ajpath.2008.070765. Epub 2008 Apr 10. Am J Pathol. 2008. PMID: 18403597 Free PMC article.
-
Role of UCP2 in the protective effects of PPARβ/δ activation on lipopolysaccharide-induced endothelial dysfunction.Biochem Pharmacol. 2016 Jun 15;110-111:25-36. doi: 10.1016/j.bcp.2016.05.004. Epub 2016 May 12. Biochem Pharmacol. 2016. PMID: 27179975
-
Chronic cigarette smoke exposure triggers a vicious cycle of leukocyte and endothelial-mediated oxidant stress that results in vascular dysfunction.Am J Physiol Heart Circ Physiol. 2020 Jul 1;319(1):H51-H65. doi: 10.1152/ajpheart.00657.2019. Epub 2020 May 15. Am J Physiol Heart Circ Physiol. 2020. PMID: 32412791 Free PMC article.
-
NADPH oxidase-dependent reactive oxygen species mediate amplified TLR4 signaling and sepsis-induced mortality in Nrf2-deficient mice.J Immunol. 2010 Jul 1;185(1):569-77. doi: 10.4049/jimmunol.0902315. Epub 2010 May 28. J Immunol. 2010. PMID: 20511556 Free PMC article.
Cited by
-
The Road to Malignant Cell Transformation after Particulate Matter Exposure: From Oxidative Stress to Genotoxicity.Int J Mol Sci. 2023 Jan 16;24(2):1782. doi: 10.3390/ijms24021782. Int J Mol Sci. 2023. PMID: 36675297 Free PMC article. Review.
-
Inhaled Pollutants: The Molecular Scene behind Respiratory and Systemic Diseases Associated with Ultrafine Particulate Matter.Int J Mol Sci. 2017 Jan 24;18(2):243. doi: 10.3390/ijms18020243. Int J Mol Sci. 2017. PMID: 28125025 Free PMC article. Review.
-
Mitochondrial genetic background modifies the relationship between traffic-related air pollution exposure and systemic biomarkers of inflammation.PLoS One. 2013 May 23;8(5):e64444. doi: 10.1371/journal.pone.0064444. Print 2013. PLoS One. 2013. PMID: 23717615 Free PMC article.
-
Effect of N-n-butyl haloperidol iodide on ROS/JNK/Egr-1 signaling in H9c2 cells after hypoxia/reoxygenation.Sci Rep. 2015 Jul 2;5:11809. doi: 10.1038/srep11809. Sci Rep. 2015. PMID: 26134032 Free PMC article.
-
Co-exposure with fullerene may strengthen health effects of organic industrial chemicals.PLoS One. 2014 Dec 4;9(12):e114490. doi: 10.1371/journal.pone.0114490. eCollection 2014. PLoS One. 2014. PMID: 25473947 Free PMC article.
References
-
- Barnoya J, Glantz SA. Cardiovascular effects of secondhand smoke: nearly as large as smoking. Circulation. 2005;111:2648–2698. - PubMed
-
- Bercher R, Bucht A, Ovrevik J, Hongslo JK, Dahlman HJ, Samuelsen JT, Schwarze PE. Involvement of NADPH oxidase and iNOS in rodent pulmonary cytokine responses to urban air and mineral particles. Inhal.Toxicol. 2007;19:645–655. - PubMed
-
- Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore! Trends Biochem. Sci. 2003;28:502–508. - PubMed
-
- Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:233–1244. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

