Inflammation in Polycystic Ovary Syndrome: underpinning of insulin resistance and ovarian dysfunction
- PMID: 22178787
- PMCID: PMC3309040
- DOI: 10.1016/j.steroids.2011.12.003
Inflammation in Polycystic Ovary Syndrome: underpinning of insulin resistance and ovarian dysfunction
Abstract
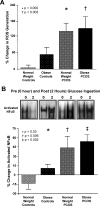
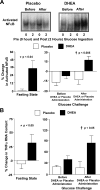
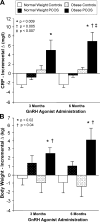
Chronic low-grade inflammation has emerged as a key contributor to the pathogenesis of Polycystic Ovary Syndrome (PCOS). A dietary trigger such as glucose is capable of inciting oxidative stress and an inflammatory response from mononuclear cells (MNC) of women with PCOS, and this phenomenon is independent of obesity. This is important because MNC-derived macrophages are the primary source of cytokine production in excess adipose tissue, and also promote adipocyte cytokine production in a paracrine fashion. The proinflammatory cytokine tumor necrosis factor-α (TNFα) is a known mediator of insulin resistance. Glucose-stimulated TNFα release from MNC along with molecular markers of inflammation are associated with insulin resistance in PCOS. Hyperandrogenism is capable of activating MNC in the fasting state, thereby increasing MNC sensitivity to glucose; and this may be a potential mechanism for promoting diet-induced inflammation in PCOS. Increased abdominal adiposity is prevalent across all weight classes in PCOS, and this inflamed adipose tissue contributes to the inflammatory load in the disorder. Nevertheless, glucose ingestion incites oxidative stress in normal weight women with PCOS even in the absence of increased abdominal adiposity. In PCOS, markers of oxidative stress and inflammation are highly correlated with circulating androgens. Chronic suppression of ovarian androgen production does not ameliorate inflammation in normal weight women with the disorder. Furthermore, in vitro studies have demonstrated the ability of pro-inflammatory stimuli to upregulate the ovarian theca cell steroidogenic enzyme responsible for androgen production. These findings support the contention that inflammation directly stimulates the polycystic ovary to produce androgens.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
References
-
- The Rotterdam ESHRE/ASRM-Sponsored PCOS Conference Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. - PubMed
-
- Eisner JR, Barnett MA, Dumesic DA, Abbott DH. Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;1:167–72. - PubMed
-
- Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of the cardiovascular risk and prevention of cardiovascular disease in women with polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95:2038–49. - PubMed
-
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;96:456–88. - PubMed
-
- González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor kB triggers inflammation and insulin resistance in polycystic ovary syndrome. Journal of Clin Endocrinol Metab. 2006;91:1508–12. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

