An agr quorum sensing system that regulates granulose formation and sporulation in Clostridium acetobutylicum
- PMID: 22179241
- PMCID: PMC3273008
- DOI: 10.1128/AEM.06376-11
An agr quorum sensing system that regulates granulose formation and sporulation in Clostridium acetobutylicum
Abstract
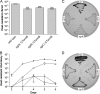
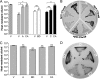
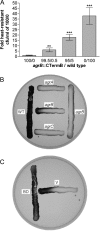
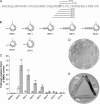
The Gram-positive, anaerobic, endospore-forming bacterium Clostridium acetobutylicum has considerable biotechnological potential due to its ability to produce solvents as fermentation products, in particular the biofuel butanol. Its genome contains a putative agr locus, agrBDCA, known in staphylococci to constitute a cyclic peptide-based quorum sensing system. In staphylococci, agrBD is required for the generation of a peptide signal that, upon extracellular accumulation, is sensed by an agrCA-encoded two-component system. Using ClosTron technology, agrB, agrC, and agrA mutants of C. acetobutylicum ATCC 824 were generated and phenotypically characterized. Mutants and wild type displayed similar growth kinetics and no apparent differences in solvent formation under the conditions tested. However, the number of heat-resistant endospores formed by the mutants in liquid culture was reduced by about one order of magnitude. On agar-solidified medium, spore formation was more strongly affected, particularly in agrA and agrC mutants. Similarly, accumulation of the starch-like storage compound granulose was almost undetectable in colonies of agrB, agrA, and agrC mutants. Importantly, these defects could be genetically complemented, demonstrating that they were directly linked to agr inactivation. A diffusible factor produced by agrBD-expressing strains was found to restore granulose and spore formation in the agrB mutant. Furthermore, a synthetic cyclic peptide, designed on the basis of the C. acetobutylicum AgrD sequence, was also capable of complementing the defects of the agrB mutant when added exogenously to the culture. Together, these findings support the hypothesis that agr-dependent quorum sensing is involved in the regulation of sporulation and granulose formation in C. acetobutylicum.
Figures


References
-
- Dürre P, Hollergschwandner C. 2004. Initiation of endospore formation in Clostridium acetobutylicum. Anaerobe 10:69–74 - PubMed
-
- Dürre P. 2005. Formation of solvents in clostridia, p 671–693 In Dürre P. (ed), Handbook on clostridia. CRC Press, Boca Raton, FL
Publication types
MeSH terms
Substances
Grants and funding
- BB/I004475/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/I004475M/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/G016224/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F003390/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

