Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor
- PMID: 22179566
- PMCID: PMC3293943
- DOI: 10.1038/nnano.2011.223
Intracellular recordings of action potentials by an extracellular nanoscale field-effect transistor
Abstract
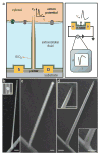
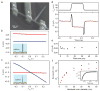
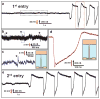
The ability to make electrical measurements inside cells has led to many important advances in electrophysiology. The patch clamp technique, in which a glass micropipette filled with electrolyte is inserted into a cell, offers both high signal-to-noise ratio and temporal resolution. Ideally, the micropipette should be as small as possible to increase the spatial resolution and reduce the invasiveness of the measurement, but the overall performance of the technique depends on the impedance of the interface between the micropipette and the cell interior, which limits how small the micropipette can be. Techniques that involve inserting metal or carbon microelectrodes into cells are subject to similar constraints. Field-effect transistors (FETs) can also record electric potentials inside cells, and because their performance does not depend on impedance, they can be made much smaller than micropipettes and microelectrodes. Moreover, FET arrays are better suited for multiplexed measurements. Previously, we have demonstrated FET-based intracellular recording with kinked nanowire structures, but the kink configuration and device design places limits on the probe size and the potential for multiplexing. Here, we report a new approach in which a SiO2 nanotube is synthetically integrated on top of a nanoscale FET. This nanotube penetrates the cell membrane, bringing the cell cytosol into contact with the FET, which is then able to record the intracellular transmembrane potential. Simulations show that the bandwidth of this branched intracellular nanotube FET (BIT-FET) is high enough for it to record fast action potentials even when the nanotube diameter is decreased to 3 nm, a length scale well below that accessible with other methods. Studies of cardiomyocyte cells demonstrate that when phospholipid-modified BIT-FETs are brought close to cells, the nanotubes can spontaneously penetrate the cell membrane to allow the full-amplitude intracellular action potential to be recorded, thus showing that a stable and tight seal forms between the nanotube and cell membrane. We also show that multiple BIT-FETs can record multiplexed intracellular signals from both single cells and networks of cells.
Conflict of interest statement
Figures

Comment in
-
A 'nano' era for electrophysiology.Nat Methods. 2012 Apr;9(4):321. doi: 10.1038/nmeth.1961. Nat Methods. 2012. PMID: 22563598 No abstract available.
References
-
- Sakmann B, Neher E. Patch clamp techniques for studying ionic channels in excitable membranes. Ann Rev Physiol. 1984;46:455–472. - PubMed
-
- Molleman A. Patch clamping: an introductory guide to patch clamp electrophysiology. Wiley; Chichester, England: 2003.
-
- Rutten WLC. Selective electrical interfaces with the nervous system. Annu Rev Biomed Eng. 2002;4:407–452. - PubMed
-
- Purves RD. Microelectrode methods for intracellular recording and ionophoresis. Academic Press; London: 1981.
-
- Chorev E, Epsztein J, Houweling AR, Lee AK, Brecht M. Electrophysiological recordings from behaving animals-going beyond spikes. Curr Opin Neurobiol. 2009;19:513–519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

