Role of cyclophilin A from brains of prion-infected mice in stimulation of cytokine release by microglia and astroglia in vitro
- PMID: 22179611
- PMCID: PMC3281647
- DOI: 10.1074/jbc.M111.269480
Role of cyclophilin A from brains of prion-infected mice in stimulation of cytokine release by microglia and astroglia in vitro
Abstract
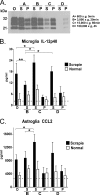
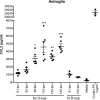
Prion diseases or transmissible spongiform encephalopathy diseases are typically characterized by deposition of abnormally folded partially protease-resistant host-derived prion protein (PrPres), which is associated with activated glia and increased release of cytokines. This neuroinflammatory response may play a role in transmissible spongiform encephalopathy pathogenesis. We previously reported that brain homogenates from prion-infected mice induced cytokine protein release in primary astroglial and microglial cell cultures. Here we measured cytokine release by cultured glial cells to determine what factors in infected brain contributed to activation of microglia and astroglia. In assays analyzing IL-12p40 and CCL2 (MCP-1), glial cells were not stimulated in vitro by either PrPres purified from infected mouse brains or prion protein amyloid fibrils produced in vitro. However, significant glial stimulation was induced by clarified scrapie brain homogenates lacking PrPres. This stimulation was greatly reduced both by antibody to cyclophilin A (CyPA), a known mediator of inflammation in peripheral tissues, and by cyclosporine A, a CyPA inhibitor. In biochemical studies, purified truncated CyPA fragments stimulated a pattern of cytokine release by microglia and astroglia similar to that induced by scrapie-infected brain homogenates, whereas purified full-length CyPA was a poor stimulator. This requirement for CyPA truncation was not reported in previous studies of stimulation of peripheral macrophages, endothelial cell cardiomyocytes, and vascular smooth muscle cells. Therefore, truncated CyPA detected in brain following prion infection may have an important role in the activation of brain-derived primary astroglia and microglia in prion disease and perhaps other neurodegenerative or neuroinflammatory diseases.
Figures






References
-
- Prusiner S. B. (1991) Molecular biology of prion diseases. Science 252, 1515–1522 - PubMed
-
- Burwinkel M., Riemer C., Schwarz A., Schultz J., Neidhold S., Bamme T., Baier M. (2004) Role of cytokines and chemokines in prion infections of the central nervous system. Int. J. Dev. Neurosci. 22, 497–505 - PubMed
-
- Ransohoff R. M., Perry V. H. (2009) Microglial physiology: unique stimuli, specialized responses. Annu. Rev. Immunol. 27, 119–145 - PubMed
-
- Brown G. C., Neher J. J. (2010) Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol. Neurobiol. 41, 242–247 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

