Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels
- PMID: 22179698
- PMCID: PMC3280559
- DOI: 10.1038/emboj.2011.465
Identification and characterization of a resident vascular stem/progenitor cell population in preexisting blood vessels
Abstract
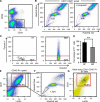
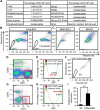
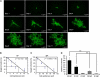
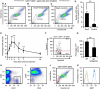
Vasculogenesis, the in-situ assembly of angioblast or endothelial progenitor cells (EPCs), may persist into adult life, contributing to new blood vessel formation. However, EPCs are scattered throughout newly developed blood vessels and cannot be solely responsible for vascularization. Here, we identify an endothelial progenitor/stem-like population located at the inner surface of preexisting blood vessels using the Hoechst method in which stem cell populations are identified as side populations. This population is dormant in the steady state but possesses colony-forming ability, produces large numbers of endothelial cells (ECs) and when transplanted into ischaemic lesions, restores blood flow completely and reconstitutes de-novo long-term surviving blood vessels. Moreover, although surface markers of this population are very similar to conventional ECs, and they reside in the capillary endothelium sub-population, the gene expression profile is completely different. Our results suggest that this heterogeneity of stem-like ECs will lead to the identification of new targets for vascular regeneration therapy.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T (2004) Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118: 149–161 - PubMed
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275: 964–967 - PubMed
-
- Boisset JC, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C (2010) In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464: 116–120 - PubMed
-
- Bruscia EM, Ziegler EC, Price JE, Weiner S, Egan ME, Krause DS (2006) Engraftment of donor-derived epithelial cells in multiple organs following bone marrow transplantation into newborn mice. Stem Cells 24: 2299–2308 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

