The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity
- PMID: 22179809
- PMCID: PMC3266411
- DOI: 10.2337/db11-0649
The L-α-lysophosphatidylinositol/GPR55 system and its potential role in human obesity
Abstract
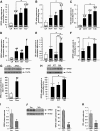
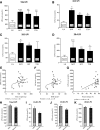
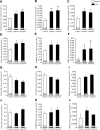
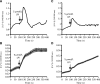
GPR55 is a putative cannabinoid receptor, and l-α-lysophosphatidylinositol (LPI) is its only known endogenous ligand. We investigated 1) whether GPR55 is expressed in fat and liver; 2) the correlation of both GPR55 and LPI with several metabolic parameters; and 3) the actions of LPI on human adipocytes. We analyzed CB1, CB2, and GPR55 gene expression and circulating LPI levels in two independent cohorts of obese and lean subjects, with both normal or impaired glucose tolerance and type 2 diabetes. Ex vivo experiments were used to measure intracellular calcium and lipid accumulation. GPR55 levels were augmented in the adipose tissue of obese subjects and further so in obese patients with type 2 diabetes when compared with nonobese subjects. Visceral adipose tissue GPR55 correlated positively with weight, BMI, and percent fat mass, particularly in women. Hepatic GPR55 gene expression was similar in obese and type 2 diabetic subjects. Circulating LPI levels were increased in obese patients and correlated with fat percentage and BMI in women. LPI increased the expression of lipogenic genes in visceral adipose tissue explants and intracellular calcium in differentiated visceral adipocytes. These findings indicate that the LPI/GPR55 system is positively associated with obesity in humans.
Figures

References
-
- Badman MK, Flier JS. The adipocyte as an active participant in energy balance and metabolism. Gastroenterology 2007;132:2103–2115 - PubMed
-
- Fernández-Real JM, Ricart W. Insulin resistance and chronic cardiovascular inflammatory syndrome. Endocr Rev 2003;24:278–301 - PubMed
-
- Matias I, Gonthier MP, Orlando P, et al. Regulation, function, and dysregulation of endocannabinoids in models of adipose and beta-pancreatic cells and in obesity and hyperglycemia. J Clin Endocrinol Metab 2006;91:3171–3180 - PubMed
-
- Pagotto U, Marsicano G, Cota D, Lutz B, Pasquali R. The emerging role of the endocannabinoid system in endocrine regulation and energy balance. Endocr Rev 2006;27:73–100 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

