Deubiquitination of EGFR by Cezanne-1 contributes to cancer progression
- PMID: 22179831
- PMCID: PMC3326441
- DOI: 10.1038/onc.2011.587
Deubiquitination of EGFR by Cezanne-1 contributes to cancer progression
Abstract
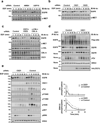
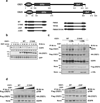
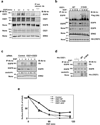
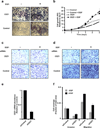
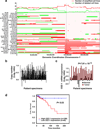
Once stimulated, the epidermal growth factor receptor (EGFR) undergoes self-phosphorylation, which, on the one hand, instigates signaling cascades, and on the other hand, recruits CBL ubiquitin ligases, which mark EGFRs for degradation. Using RNA interference screens, we identified a deubiquitinating enzyme, Cezanne-1, that opposes receptor degradation and enhances EGFR signaling. These functions require the catalytic- and ubiquitin-binding domains of Cezanne-1, and they involve physical interactions and transphosphorylation of Cezanne-1 by EGFR. In line with the ability of Cezanne-1 to augment EGF-induced growth and migration signals, the enzyme is overexpressed in breast cancer. Congruently, the corresponding gene is amplified in approximately one third of mammary tumors, and high transcript levels predict an aggressive disease course. In conclusion, deubiquitination by Cezanne-1 curtails degradation of growth factor receptors, thereby promotes oncogenic growth signals.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Avraham R, Yarden Y. Feedback regulation of EGFR signalling: decision making by early and delayed loops. Nat Rev Mol Cell Biol. 2010;12:104–117. - PubMed
-
- Bowers K, Piper SC, Edeling MA, Gray SR, Owen DJ, Lehner PJ, et al. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J Biol Chem. 2006;281:5094–5105. - PubMed
-
- Cheng KW, Lahad JP, Kuo WL, Lapuk A, Yamada K, Auersperg N, et al. The RAB25 small GTPase determines aggressiveness of ovarian and breast cancers. Nat Med. 2004;10:1251–1256. - PubMed
-
- Courbard JR, Fiore F, Adelaide J, Borg JP, Birnbaum D, Ollendorff V. Interaction between two ubiquitin-protein isopeptide ligases of different classes, CBLC and AIP4/ITCH. J Biol Chem. 2002;277:45267–45275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

