Does using autograft bone chips achieve consistent bone ingrowth in primary TKA?
- PMID: 22179980
- PMCID: PMC3369085
- DOI: 10.1007/s11999-011-2214-2
Does using autograft bone chips achieve consistent bone ingrowth in primary TKA?
Abstract
Background: Cementless fixation remains controversial in TKA due to the challenge of achieving consistent skeletal attachment. Factors predicting durable fixation are not clearly understood, but we presumed bone ingrowth could be enhanced by the quantity of host bone and application of autograft bone chips.
Questions/purposes: We asked: (1) Did the amount of bone ingrowth exceed the amount of periprosthetic and host bone with the addition of autograft bone chips? (2) Did the amount of bone ingrowth increase with implantation time? And (3) did osteolysis along the porous-coated interface and screw tracts progress with implantation time?
Methods: We measured the amount of bone in the porous-coated, periprosthetic, and host bone regions in 19 postmortem retrieved cementless primary total knee implants. The amount of bone in apposition to the implant surface, and alternatively lysed bone, was analyzed radiographically to assess the progression of osteolysis.
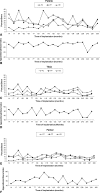
Results: While bone ingrowth tended to be less than periprosthetic and host bone in all three components, it was only significantly less in the patellar component. Bone ingrowth increased in all three components over time, but progression of osteolysis did not.
Conclusions: Even after long-term followup, the amount of bone ingrowth did not surpass host bone levels, suggesting the amount of a patient's host bone is a limiting factor in the amount of bone ingrowth achievable for this cementless design. It remains unknown whether compromised osteopenic bone could achieve the amount of bone attachment necessary to provide durable fixation over time.
Figures






Similar articles
-
Liner exchange and bone grafting: rare option to treat wear & lysis of stable TKAs.Clin Orthop Relat Res. 2011 Jan;469(1):154-9. doi: 10.1007/s11999-010-1521-3. Clin Orthop Relat Res. 2011. PMID: 20809171 Free PMC article.
-
Bone ingrowth into porous-coated tibial components implanted with autograft bone chips. Analysis of ten consecutively retrieved implants.J Arthroplasty. 1992 Dec;7(4):483-93. doi: 10.1016/s0883-5403(06)80069-0. J Arthroplasty. 1992. PMID: 1479367
-
Osseointegration in porous coated knee arthroplasty. The influence of component coating type in sheep.Acta Orthop Scand Suppl. 1999 Oct;288:1-35. Acta Orthop Scand Suppl. 1999. PMID: 10949554
-
Cementless fixation in total knee arthroplasty: past, present, and future.J Knee Surg. 2008 Oct;21(4):307-14. doi: 10.1055/s-0030-1247837. J Knee Surg. 2008. PMID: 18979934 Review.
-
Bone Defects in Revision Total Knee Arthroplasty and Management.Orthop Surg. 2019 Feb;11(1):15-24. doi: 10.1111/os.12425. Epub 2019 Feb 27. Orthop Surg. 2019. PMID: 30809942 Free PMC article. Review.
Cited by
-
Treatment for Wear and Osteolysis in Well-Fixed Uncemented TKR.ISRN Orthop. 2013 Feb 11;2013:398298. doi: 10.1155/2013/398298. eCollection 2013. ISRN Orthop. 2013. PMID: 24959358 Free PMC article.
-
Finite element analysis of the tibial bone graft in cementless total knee arthroplasty.J Orthop Surg Res. 2018 May 16;13(1):113. doi: 10.1186/s13018-018-0830-1. J Orthop Surg Res. 2018. PMID: 29769146 Free PMC article.
-
50 years of scanning electron microscopy of bone-a comprehensive overview of the important discoveries made and insights gained into bone material properties in health, disease, and taphonomy.Bone Res. 2019 May 22;7:15. doi: 10.1038/s41413-019-0053-z. eCollection 2019. Bone Res. 2019. PMID: 31123620 Free PMC article.
References
-
- Bischoff UW, Freeman MA, Smith D, Tuke MA, Gregson PJ. Wear induced by motion between bone and titanium or cobalt-chrome alloys. J Bone Joint Surg Br. 1994;76:713–716. - PubMed
-
- Bloebaum RD, Bachus KN, Jensen JW, Scott DF, Hofmann AA. Porous-coated metal-backed patellar components in total knee replacement. J Bone Joint Surg Am. 1998;80:518–528. - PubMed
-
- Bloebaum RD, Merrell M, Gustke K, Simmons M. Retrieval analysis of a hydroxyapatite-coated hip prosthesis. Clin Orthop Relat Res. 1991;267:97–102. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

