IL-11 is a parietal cell cytokine that induces atrophic gastritis
- PMID: 22180059
- PMCID: PMC3471558
- DOI: 10.1136/gutjnl-2011-300539
IL-11 is a parietal cell cytokine that induces atrophic gastritis
Abstract
Background and aims: IL-is important in gastric damage, mucosal repair and gastric cancer progression. We analysed IL-11 expression in H.pylori infected mouse stomach, the site of gastric IL-11 expression in mice and humans, and the effect of exogenous IL-11 on gastric mucosal homeostasis.
Methods: IL-11 protein was localised in mouse and human stomach. The impact of chronic, exogenous IL-11 on normal mouse stomach was examined histologically and transcriptionally by microarray, confirmed by mRNA and protein analysis. Functional impact of IL-11 on gastric acid secretion was determined.
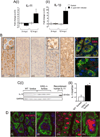
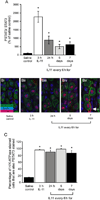
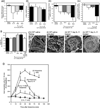
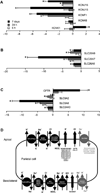
Results: In mice infected with H.pylori, IL-11 was increased in fundic mucosa with temporal expression similar to IL-1b. IL-11 protein was localised predominantly to parietal cells in mouse and human stomach. Application of exogenous IL-11 to resulted in fundic parietal and chief cell loss, hyperplasia, mucous cell metaplasia and inflammation. Coincident with cellular changes were an increased gastric pH, altered parietal cell ultrastructure and altered gene expression, particularly genes involved in immune response and ion transport which could result in compromised acid secretion. We confirmed that a single dose of IL-11 effectively ablated the gastric response to histamine.
Conclusions: IL-11 is a parietal cell cytokine that blocks gastric acid secretion, likely via reducing expression of parietal cell ion transport genes, CCKb and histamine H2 receptors. IL-11 expression is increased in H. pylori infected mouse stomach and treatment of wild type mice with IL-11 induced changes in the gastric fundic mucosa reminiscent of chronic atrophic gastritis, a precursor to gastric cancer.
Figures


Similar articles
-
Mucosal expression of aquaporin-4 in the stomach of histamine type 2 receptor knockout mice and Helicobacter pylori-infected mice.J Gastroenterol Hepatol. 2014 Dec;29 Suppl 4:53-9. doi: 10.1111/jgh.12771. J Gastroenterol Hepatol. 2014. PMID: 25521734
-
Helicobacter pylori VacA disrupts apical membrane-cytoskeletal interactions in gastric parietal cells.J Biol Chem. 2008 Sep 26;283(39):26714-25. doi: 10.1074/jbc.M800527200. Epub 2008 Jul 14. J Biol Chem. 2008. PMID: 18625712 Free PMC article.
-
Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression.J Pathol. 2007 Oct;213(2):140-51. doi: 10.1002/path.2218. J Pathol. 2007. PMID: 17724739
-
The relationships between chronic gastritis and gastric acid secretion.Aliment Pharmacol Ther. 1996 Apr;10 Suppl 1:103-18. doi: 10.1046/j.1365-2036.1996.22164011.x. Aliment Pharmacol Ther. 1996. PMID: 8730265 Review.
-
Review article: exploring the link between Helicobacter pylori and gastric cancer.Aliment Pharmacol Ther. 1999 Mar;13 Suppl 1:3-11. doi: 10.1046/j.1365-2036.1999.00002.x. Aliment Pharmacol Ther. 1999. PMID: 10209681 Review.
Cited by
-
IL-33 Accelerates Chronic Atrophic Gastritis through AMPK-ULK1 Axis Mediated Autolysosomal Degradation of GKN1.Int J Biol Sci. 2024 Apr 8;20(6):2323-2338. doi: 10.7150/ijbs.93573. eCollection 2024. Int J Biol Sci. 2024. PMID: 38617533 Free PMC article.
-
Interleukin-17A Promotes Parietal Cell Atrophy by Inducing Apoptosis.Cell Mol Gastroenterol Hepatol. 2018 Jan 2;5(4):678-690.e1. doi: 10.1016/j.jcmgh.2017.12.012. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 29930985 Free PMC article.
-
Optimized therapeutic potential of Sijunzi-similar formulae for chronic atrophic gastritis via Bayesian network meta-analysis.EXCLI J. 2024 Sep 6;23:1185-1207. doi: 10.17179/excli2024-7618. eCollection 2024. EXCLI J. 2024. PMID: 39421026 Free PMC article.
-
Morphogen Signals Shaping the Gastric Glands in Health and Disease.Int J Mol Sci. 2022 Mar 26;23(7):3632. doi: 10.3390/ijms23073632. Int J Mol Sci. 2022. PMID: 35408991 Free PMC article. Review.
-
H. Pylori-Facilitated TERT/Wnt/β-Catenin Triggers Spasmolytic Polypeptide-Expressing Metaplasia and Oxyntic Atrophy.Adv Sci (Weinh). 2025 Jan;12(3):e2401227. doi: 10.1002/advs.202401227. Epub 2024 Nov 25. Adv Sci (Weinh). 2025. PMID: 39587848 Free PMC article.
References
-
- Verdecchia A, Mariotto A, Gatta G, et al. Comparison of stomach cancer incidence and survival in four continents. Eur J Cancer. 2003;39:1603–1609. - PubMed
-
- Houghton J, Wang TC. Helicobacter pylori and gastric cancer: a new paradigm for inflammation-associated epithelial cancers. Gastroenterology. 2005;128:1567–1578. - PubMed
-
- Correa P. The biological model of gastric carcinogenesis. IARC Sci Publ. 2004;157:301–310. - PubMed
-
- Kim TY, Lee HJ, Hwang KS, et al. Methylation of RUNX3 in various types of human cancers and premalignant stages of gastric carcinoma. Lab Invest. 2004;84:479–484. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
