A conserved RpoS-dependent small RNA controls the synthesis of major porin OmpD
- PMID: 22180532
- PMCID: PMC3333887
- DOI: 10.1093/nar/gkr1156
A conserved RpoS-dependent small RNA controls the synthesis of major porin OmpD
Abstract
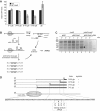
A remarkable feature of many small non-coding RNAs (sRNAs) of Escherichia coli and Salmonella is their accumulation in the stationary phase of bacterial growth. Several stress response regulators and sigma factors have been reported to direct the transcription of stationary phase-specific sRNAs, but a widely conserved sRNA gene that is controlled by the major stationary phase and stress sigma factor, σ(S) (RpoS), has remained elusive. We have studied in Salmonella the conserved SdsR sRNA, previously known as RyeB, one of the most abundant stationary phase-specific sRNAs in E. coli. Alignments of the sdsR promoter region and genetic analysis strongly suggest that this sRNA gene is selectively transcribed by σ(S). We show that SdsR down-regulates the synthesis of the major Salmonella porin OmpD by Hfq-dependent base pairing; SdsR thus represents the fourth sRNA to regulate this major outer membrane porin. Similar to the InvR, MicC and RybB sRNAs, SdsR recognizes the ompD mRNA in the coding sequence, suggesting that this mRNA may be primarily targeted downstream of the start codon. The SdsR-binding site in ompD was localized by 3'-RACE, an experimental approach that promises to be of use in predicting other sRNA-target interactions in bacteria.
Figures

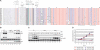

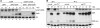


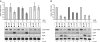
Similar articles
-
The target spectrum of SdsR small RNA in Salmonella.Nucleic Acids Res. 2016 Dec 1;44(21):10406-10422. doi: 10.1093/nar/gkw632. Epub 2016 Jul 12. Nucleic Acids Res. 2016. PMID: 27407104 Free PMC article.
-
Hfq assists small RNAs in binding to the coding sequence of ompD mRNA and in rearranging its structure.RNA. 2016 Jul;22(7):979-94. doi: 10.1261/rna.055251.115. Epub 2016 May 6. RNA. 2016. PMID: 27154968 Free PMC article.
-
Positional effects of AAN motifs in rpoS regulation by sRNAs and Hfq.J Mol Biol. 2014 Jan 23;426(2):275-85. doi: 10.1016/j.jmb.2013.08.026. Epub 2013 Sep 16. J Mol Biol. 2014. PMID: 24051417 Free PMC article.
-
Small Regulatory RNAs in the Enterobacterial Response to Envelope Damage and Oxidative Stress.Microbiol Spectr. 2018 Jul;6(4):10.1128/microbiolspec.rwr-0022-2018. doi: 10.1128/microbiolspec.RWR-0022-2018. Microbiol Spectr. 2018. PMID: 29992897 Free PMC article. Review.
-
Small regulatory bacterial RNAs regulating the envelope stress response.Biochem Soc Trans. 2017 Apr 15;45(2):417-425. doi: 10.1042/BST20160367. Biochem Soc Trans. 2017. PMID: 28408482 Free PMC article. Review.
Cited by
-
Resistance Profiles of Salmonella Isolates Exposed to Stresses and the Expression of Small Non-coding RNAs.Front Microbiol. 2020 Feb 28;11:130. doi: 10.3389/fmicb.2020.00130. eCollection 2020. Front Microbiol. 2020. PMID: 32180763 Free PMC article.
-
Canonical and non-canonical EcfG sigma factors control the general stress response in Rhizobium etli.Microbiologyopen. 2013 Dec;2(6):976-87. doi: 10.1002/mbo3.137. Epub 2013 Oct 28. Microbiologyopen. 2013. PMID: 24311555 Free PMC article.
-
Identification of bacterial sRNA regulatory targets using ribosome profiling.Nucleic Acids Res. 2015 Dec 2;43(21):10308-20. doi: 10.1093/nar/gkv1158. Epub 2015 Nov 5. Nucleic Acids Res. 2015. PMID: 26546513 Free PMC article.
-
Small RNAs and their role in biofilm formation.Trends Microbiol. 2013 Jan;21(1):39-49. doi: 10.1016/j.tim.2012.10.008. Epub 2012 Nov 20. Trends Microbiol. 2013. PMID: 23178000 Free PMC article. Review.
-
BSRD: a repository for bacterial small regulatory RNA.Nucleic Acids Res. 2013 Jan;41(Database issue):D233-8. doi: 10.1093/nar/gks1264. Epub 2012 Nov 29. Nucleic Acids Res. 2013. PMID: 23203879 Free PMC article.
References
-
- Rivas E, Klein RJ, Jones TA, Eddy SR. Computational identification of noncoding RNAs in E. coli by comparative genomics. Curr. Biol. 2001;11:1369–1373. - PubMed
-
- Chen S, Lesnik EA, Hall TA, Sampath R, Griffey RH, Ecker DJ, Blyn LB. A bioinformatics based approach to discover small RNA genes in the Escherichia coli genome. Biosystems. 2002;65:157–177. - PubMed
-
- Argaman L, Hershberg R, Vogel J, Bejerano G, Wagner EG, Margalit H, Altuvia S. Novel small RNA-encoding genes in the intergenic regions of Escherichia coli. Curr. Biol. 2001;11:941–950. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

