Pre-B cell leukemia homeobox 1 is associated with lupus susceptibility in mice and humans
- PMID: 22180614
- PMCID: PMC3253202
- DOI: 10.4049/jimmunol.1002362
Pre-B cell leukemia homeobox 1 is associated with lupus susceptibility in mice and humans
Abstract
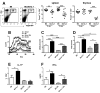
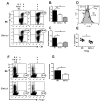
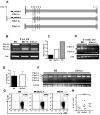
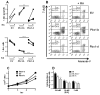
Sle1a.1 is part of the Sle1 susceptibility locus, which has the strongest association with lupus nephritis in the NZM2410 mouse model. In this study, we show that Sle1a.1 results in the production of activated and autoreactive CD4(+) T cells. Additionally, Sle1a.1 expression reduces the peripheral regulatory T cell pool, as well as induces a defective response of CD4(+) T cells to the retinoic acid expansion of TGF-β-induced regulatory T cells. At the molecular level, Sle1a.1 corresponds to an increased expression of a novel splice isoform of Pbx1, Pbx1-d. Pbx1-d overexpression is sufficient to induce an activated/inflammatory phenotype in Jurkat T cells and to decrease their apoptotic response to retinoic acid. PBX1-d is expressed more frequently in the CD4(+) T cells from lupus patients than from healthy controls, and its presence correlates with an increased central memory T cell population. These findings indicate that Pbx1 is a novel lupus susceptibility gene that regulates T cell activation and tolerance.
Conflict of interest statement
Figures

References
-
- Datta SK. Major peptide autoepitopes for nucleosome-centered T and B cell interaction in human and murine lupus. Ann NY Acad Sci. 2003;987:79–90. - PubMed
-
- Kong PL, Odegard JM, Bouzahzah F, Choi JY, Eardley LD, Zielinski CE, Craft JE. Intrinsic T cell defects in systemic autoimmunity. Ann NY Acad Sci. 2003;987:60–67. - PubMed
-
- Crispin JC, Kyttaris VC, Juang YT, Tsokos GC. How signaling and gene transcription aberrations dictate the systemic lupus erythematosus T cell phenotype. Trends Immunol. 2008;29:110–115. - PubMed
-
- La Cava A. T-regulatory cells in systemic lupus erythematosus. Lupus. 2008;17:421–425. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

