Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib
- PMID: 22180633
- PMCID: PMC3276477
- DOI: 10.1194/jlr.M018010
Changes in lipoprotein subfraction concentration and composition in healthy individuals treated with the CETP inhibitor anacetrapib
Abstract
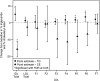
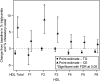
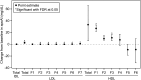
We investigated the effects of the cholesteryl ester (CE) transfer protein inhibitor anacetrapib (ANA) on plasma lipids, lipoprotein subfraction concentrations, and lipoprotein composition in 30 healthy individuals. Participants (n = 30) were randomized to ANA 20 mg/day, 150 mg/day, or placebo for 2 weeks. Changes in concentration of lipoprotein subfractions were assessed using ion mobility, and compositional analyses were performed on fractions separated by density gradient ultracentrifugation. ANA 150 mg/day versus placebo resulted in significant decreases in LDL-cholesterol (26%) and apo B (29%) and increases in HDL-cholesterol (82%). Concentrations of medium and small VLDL, large intermediate density lipoprotein (IDL), and medium and small LDL (LDL2a, 2b, and 3a) decreased whereas levels of very small and dense LDL4b were increased. There was enrichment of triglycerides and reduction of CE in VLDL, IDL, and the densest LDL fraction. Levels of large buoyant HDL particles were substantially increased, and there was enrichment of CE, apo AI, and apoCIII, but not apoAII or apoE, in the mid-HDL density range. Changes in lipoprotein subfraction concentrations and composition with ANA 20 mg/day were similar to those for ANA 150 mg/day but were generally smaller in magnitude. The impact of these changes on cardiovascular risk remains to be determined.
Trial registration: ClinicalTrials.gov NCT01252953.
Figures
References
-
- Pepine C. J. 2010. Residual risk for secondary ischemic events in patients with atherothrombotic disease: opportunity for future improvements in patient care. Ann. Med. 42: 19–35. - PubMed
-
- Tall A. R. 1993. Plasma cholesteryl ester transfer protein. J. Lipid Res. 34: 1255–1274. - PubMed
-
- Koizumi J., Mabuchi H., Yoshimura A., Michishita I., Takeda M., Itoh H., Sakai Y., Sakai T., Ueda K., Takeda R. 1985. Deficiency of serum cholesteryl-ester transfer activity in patients with familial hyperalphalipoproteinaemia. Atherosclerosis. 58: 175–186. - PubMed
-
- Thompson A., Di Angelantonio E., Sarwar N., Erqou S., Saleheen D., Dullaart R. P., Keavney B., Ye Z., Danesh J. 2008. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA. 299: 2777–2788. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

