Tandem double intramolecular [4+2]/[3+2] cycloadditions of nitroalkenes: construction of the pentacyclic core structure of daphnilactone B
- PMID: 22180670
- PMCID: PMC3238388
- DOI: 10.1016/j.tet.2009.05.060
Tandem double intramolecular [4+2]/[3+2] cycloadditions of nitroalkenes: construction of the pentacyclic core structure of daphnilactone B
Abstract
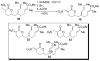
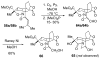
An asymmetric synthesis of the ABCD ring system of daphnilactone B is described. The synthesis features a tandem, double intramolecular, [4+2]/[3+2] cycloaddition of a highly functionalized, enantiomerically enriched nitroalkene to generate a pentacyclic nitroso acetal. The cycloaddition establishes six contiguous stereogenic centers including the critical CD ring junction that bears two quaternary stereogenic centers. Hydrogenolysis of the nitroso acetal followed by amide reduction and cyclization provided the AB rings. The methyl substituent on the A ring was installed in the correct configuration via hydrogenation of an exocyclic olefin in the final step.
Figures




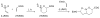

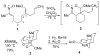
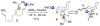
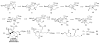



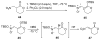


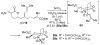

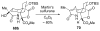


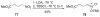

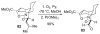
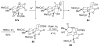


References
-
- Heathcock CH. Angew Chem, Int Ed Engl. 1992;31:665–681.
-
- Sakabe N, Hirata Y. Tetrahedron Lett. 1966;7:965–968.
-
- Sakurai N, Sakabe N, Hirata Y. Tetrahedron Lett. 1966;7:6309–6314.
-
- Toda M, Yamamura S, Hirata Y. Tetrahedron Lett. 1969;10:2585–2586.
-
- Sasaki K, Hirata Y. J Chem Soc B. 1971:1565–1568.
Grants and funding
LinkOut - more resources
Full Text Sources
