DNA binding and unwinding by self-assembled supramolecular hetero-bimetallacycles
- PMID: 22180697
- PMCID: PMC3237687
- DOI: 10.1021/om200802v
DNA binding and unwinding by self-assembled supramolecular hetero-bimetallacycles
Abstract
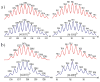
Two new tetracationic hetero-bimetallacycles were prepared from a bis-pyridine amide ligand and metal (Pd and Pt) acceptors. We found that both self-assembled hetero-bimetallacycles bind and unwind supercoiled DNA as established by photophysical and gel electrophoresis analyses, respectively.
Figures
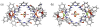



References
-
- Lehn J-M. Supramolecular Chemistry, concepts and perspectiVes. VCH; New York: 1995.
- Steed JW, Atwood JL. Supramolecular Chemistry. VCH; New York: 2000.
- Leininger S, Olenyuk B, Stang PJ. Chem Rev. 2000;100:853. - PubMed
- Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:369. - PubMed
- Fiedler D, Leung DH, Bergman RG, Raymond KN. Acc Chem Res. 2005;38:349. - PubMed
- Oliver CG, Ulman PA, Wiester MJ, Mirkin CA. Acc Chem Res. 2008;41:1618. - PMC - PubMed
- Raymond KN. Nature. 2009;460:585.
- Pluth MD, Bergman RG, Raymond KN. Acc Chem Res. 2009;42:1650. - PubMed
- Lee J, Farha OK, Roberts J, Scheidt KA, Nguyen ST, Hupp JT. Chem Soc Rev. 2009;38:1450. - PubMed
- Pluth MD, Bergman RG, Raymond KN. J Am Chem Soc. 2008;130:6362. - PubMed
- Klosterman JK, Yamauchi Y, Fujita M. Chem Soc Rev. 2009;38:1714. - PubMed
- Severin K. Chem Commun. 2006:3859. - PubMed
-
- Northrop BH, Zheng YR, Chi KW, Stang PJ. Acc Chem Res. 2009;42:1554. - PMC - PubMed
- Zheng YR, Yang HB, Northrop BH, Ghosh K, Stang PJ. Inorg Chem. 2008;47:4706. - PubMed
- Zheng YR, Yang HB, Ghosh K, Zhao L, Stang PJ. Chem Eur J. 2009;15:7203. - PMC - PubMed
- Seidel SR, Stang PJ. Acc Chem Res. 2002;35:972. - PubMed
- Ghosh S, Mukherjee PS. Inorg Chem. 2009;48:2605. - PubMed
- Shanmugaraju S, Bar AK, Chi KW, Mukherjee PS. Organometallics. 2010;29:2971.
- Vajpayee V, Kim H, Mishra A, Mukherjee PS, Stang PJ, Lee MH, Kim HK, Chi KW. Dalton Trans. 2011;40:3112. - PMC - PubMed
-
- Stang PJ, Olenyuk B. Acc Chem Res. 1997;30:502.
- Northrop BH, Yang HB, Stang PJ. Chem Commun. 2008:5896. - PMC - PubMed
- Fujita M, Tominaga M, Hori A, Therrien B. Acc Chem Res. 2005;38:369. - PubMed
- Ghosh S, Mukherjee PS. Inorg Chem. 2009;48:549. - PubMed
- Bar AK, Chakrabarty R, Mostafa G, Mukherjee PS. Angew Chem, Int Ed. 2008;47:8455. - PubMed
- Ghosh S, Mukherjee PS. J Org Chem. 2006;71:8412. - PubMed
- Dash BP, Satapathy R, Maguire JA, Hosmane NS. Org Lett. 2008;10:2247. - PubMed
- Shanmugaraju S, Bar AK, Chi K-W, Mukherjee PS. Organometallics. 2010;29:2971.
-
- Barbour LJ, Orr GW, Atwood JL. Nature. 1998;393
- Qin Z, Jennings MC, Puddephatt RJ. Chem Commun. 2001:2676.
- Qin Z, Jennings MC, Puddephatt RJ. Chem Commun. 2002:354. - PubMed
- Qin Z, Jennings MC, Puddephatt RJ. Inorg Chem. 2003;42:1956. - PubMed
- Mishra A, Ali A, Upreti S, Whittingham MS, Gupta R. Inorg Chem. 2009;48:5234. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
