Low-volume plus ascorbic acid vs high-volume plus simethicone bowel preparation before colonoscopy
- PMID: 22180711
- PMCID: PMC3233675
- DOI: 10.3748/wjg.v17.i42.4689
Low-volume plus ascorbic acid vs high-volume plus simethicone bowel preparation before colonoscopy
Abstract
Aim: To investigate the effectiveness of low-volume plus ascorbic acid [polyethylene glycol plus ascorbic acid (PEG + Asc)] and high-volume plus simethicone [polyethylene glycol plus simethicone (PEG + Sim)] bowel preparations.
Methods: A total of one hundred and forty-four outpatients (76 males), aged from 20 to 84 years (median age 59.5 years), who attended our Department, were divided into two groups, age and sex matched, and underwent colonoscopy. Two questionnaires, one for patients reporting acceptability and the other for endoscopists evaluating bowel cleansing effectiveness according to validated scales, were completed. Indications, timing of examination and endoscopical findings were recorded. Biopsy forceps were used as a measuring tool in order to determine polyp endoscopic size estimation. Difficulty in completing the preparation was rated in a 5-point Likert scale (1 = easy to 5 = unable). Adverse experiences (fullness, cramps, nausea, vomiting, abdominal pain, headache and insomnia), number of evacuations and types of activities performed during preparation (walking or resting in bed) were also investigated.
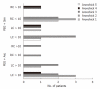
Results: Seventy-two patients were selected for each group. The two groups were age and sex matched as well as being comparable in terms of medical history and drug therapies taken. Fourteen patients dropped out from the trial because they did not complete the preparation procedure. Ratings of global bowel cleansing examinations were considered to be adequate in 91% of PEG + Asc and 88% of PEG + Sim patients. Residual Stool Score indicated similar levels of amount and consistency of residual stool; there was a significant difference in the percentage of bowel wall visualization in favour of PEG + Sim patients. In the PEG + Sim group, 12 adenomas ≤ 10 mm diameter (5/left colon + 7/right colon) vs 9 (8/left colon + 1/right colon) in the PEG + Asc group were diagnosed. Visualization of small lesions seems to be one of the primary advantages of the PEG + Sim preparation.
Conclusion: PEG + Asc is a good alternative solution as a bowel preparation but more improvements are necessary in order to achieve the target of a perfect preparation.
Keywords: Ascorbic acid; Bowel preparation; Colonoscopy; Polyethylene glycol; Simethicone.
Figures

References
-
- Arditi C, Peytremann-Bridevaux I, Burnand B, Eckardt VF, Bytzer P, Agréus L, Dubois RW, Vader JP, Froehlich F, Pittet V, Schusselé Filliettaz S, Juillerat P, Gonvers JJ. Appropriateness of colonoscopy in Europe (EPAGE II). Screening for colorectal cancer. Endoscopy. 2009;41:200–208. - PubMed
-
- Nelson RS, Thorson AG. Colorectal cancer screening. Curr Oncol Rep. 2009;11:482–489. - PubMed
-
- Tsikitis VL, Malireddy K, Green EA, Christensen B, Whelan R, Hyder J, Marcello P, Larach S, Lauter D, Sargent DJ, et al. Postoperative surveillance recommendations for early stage colon cancer based on results from the clinical outcomes of surgical therapy trial. J Clin Oncol. 2009;27:3671–3676. - PMC - PubMed
-
- Bitoun A, Ponchon T, Barthet M, Coffin B, Dugué C, Halphen M. Results of a prospective randomised multicentre controlled trial comparing a new 2-L ascorbic acid plus polyethylene glycol and electrolyte solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment Pharmacol Ther. 2006;24:1631–1642. - PubMed
-
- Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, Hoffman B, Jacobson BC, Mergener K, Petersen BT, et al. Quality indicators for colonoscopy. Gastrointest Endosc. 2006;63:S16–S28. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

