Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience
- PMID: 22180766
- PMCID: PMC3237675
- DOI: 10.1016/j.coviro.2011.10.017
Natural killer cell responses during viral infections: flexibility and conditioning of innate immunity by experience
Abstract
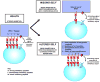
Natural killer (NK) cells mediate innate defense against viral infections, but the mechanisms in place to access their functions as needed during diverse challenges while limiting collateral damage are poorly understood. Recent molecular characterization of effects mediated through infection-induced inhibitory/activating NK receptor-ligand pairs and cytokines are providing new insights into pathways regulating their responses and revealing unexpected consequences for NK cell subset effects, maintenance, proliferation and function through times overlapping with adaptive and long-lived immunity. The observations define flexible pathways for experience-induced 'conditioning' and challenge narrowly defined roles for NK cells and innate immunity as first responders with prescribed functions. They suggest that individual experiences as well as genes influence the innate immune resources available to fight off an infection.
Figures




References
-
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–510. - PubMed
-
- Biron CA, Nguyen KB, Pien GC, Cousens LP, Salazar-Mather TP. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu Rev Immunol. 1999;17:189–220. - PubMed
-
- Orange JS, Ballas ZK. Natural killer cells in human health and disease. Clin Immunol. 2006;118:1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

