Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue
- PMID: 22180785
- PMCID: PMC3236741
- DOI: 10.1371/journal.pone.0028501
Chymase-dependent generation of angiotensin II from angiotensin-(1-12) in human atrial tissue
Abstract
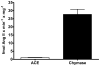
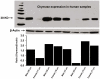
Since angiotensin-(1-12) [Ang-(1-12)] is a non-renin dependent alternate precursor for the generation of cardiac Ang peptides in rat tissue, we investigated the metabolism of Ang-(1-12) by plasma membranes (PM) isolated from human atrial appendage tissue from nine patients undergoing cardiac surgery for primary control of atrial fibrillation (MAZE surgical procedure). PM was incubated with highly purified ¹²⁵I-Ang-(1-12) at 37°C for 1 h with or without renin-angiotensin system (RAS) inhibitors [lisinopril for angiotensin converting enzyme (ACE), SCH39370 for neprilysin (NEP), MLN-4760 for ACE2 and chymostatin for chymase; 50 µM each]. ¹²⁵I-Ang peptide fractions were identified by HPLC coupled to an inline γ-detector. In the absence of all RAS inhibitor, ¹²⁵I-Ang-(1-12) was converted into Ang I (2±2%), Ang II (69±21%), Ang-(1-7) (5±2%), and Ang-(1-4) (2±1%). In the absence of all RAS inhibitor, only 22±10% of ¹²⁵I-Ang-(1-12) was unmetabolized, whereas, in the presence of the all RAS inhibitors, 98±7% of ¹²⁵I-Ang-(1-12) remained intact. The relative contribution of selective inhibition of ACE and chymase enzyme showed that ¹²⁵I-Ang-(1-12) was primarily converted into Ang II (65±18%) by chymase while its hydrolysis into Ang II by ACE was significantly lower or undetectable. The activity of individual enzyme was calculated based on the amount of Ang II formation. These results showed very high chymase-mediated Ang II formation (28±3.1 fmol × min⁻¹ × mg⁻¹, n = 9) from ¹²⁵I-Ang-(1-12) and very low or undetectable Ang II formation by ACE (1.1±0.2 fmol×min⁻¹ × mg⁻¹). Paralleling these findings, these tissues showed significant content of chymase protein that by immunocytochemistry were primarily localized in atrial cardiac myocytes. In conclusion, we demonstrate for the first time in human cardiac tissue a dominant role of cardiac chymase in the formation of Ang II from Ang-(1-12).
Conflict of interest statement
Figures



References
-
- Paul M, Poyan MA, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. - PubMed
-
- Nagata S, Kato J, Sasaki K, Minamino N, Eto T, et al. Isolation and identification of proangiotensin-12, a possible component of the renin-angiotensin system. Biochem Biophys Res Commun. 2006;350:1026–1031. - PubMed
-
- Prosser HC, Forster ME, Richards AM, Pemberton CJ. Cardiac chymase converts rat proAngiotensin-12 (PA12) to angiotensin II: effects of PA12 upon cardiac haemodynamics. Cardiovasc Res. 2009;82:40–50. - PubMed
-
- Prosser HC, Richards AM, Forster ME, Pemberton CJ. Regional vascular response to ProAngiotensin-12 (PA12) through the rat arterial system. Peptides. 2010;31:1540–1545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

