Novel insights in the fecal egg count reduction test for monitoring drug efficacy against soil-transmitted helminths in large-scale treatment programs
- PMID: 22180801
- PMCID: PMC3236725
- DOI: 10.1371/journal.pntd.0001427
Novel insights in the fecal egg count reduction test for monitoring drug efficacy against soil-transmitted helminths in large-scale treatment programs
Abstract
Background: The fecal egg count reduction test (FECRT) is recommended to monitor drug efficacy against soil-transmitted helminths (STHs) in public health. However, the impact of factors inherent to study design (sample size and detection limit of the fecal egg count (FEC) method) and host-parasite interactions (mean baseline FEC and aggregation of FEC across host population) on the reliability of FECRT is poorly understood.
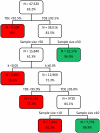
Methodology/principal findings: A simulation study was performed in which FECRT was assessed under varying conditions of the aforementioned factors. Classification trees were built to explore critical values for these factors required to obtain conclusive FECRT results. The outcome of this analysis was subsequently validated on five efficacy trials across Africa, Asia, and Latin America. Unsatisfactory (<85.0%) sensitivity and specificity results to detect reduced efficacy were found if sample sizes were small (<10) or if sample sizes were moderate (10-49) combined with highly aggregated FEC (k<0.25). FECRT remained inconclusive under any evaluated condition for drug efficacies ranging from 87.5% to 92.5% for a reduced-efficacy-threshold of 90% and from 92.5% to 97.5% for a threshold of 95%. The most discriminatory study design required 200 subjects independent of STH status (including subjects who are not excreting eggs). For this sample size, the detection limit of the FEC method and the level of aggregation of the FEC did not affect the interpretation of the FECRT. Only for a threshold of 90%, mean baseline FEC <150 eggs per gram of stool led to a reduced discriminatory power.
Conclusions/significance: This study confirms that the interpretation of FECRT is affected by a complex interplay of factors inherent to both study design and host-parasite interactions. The results also highlight that revision of the current World Health Organization guidelines to monitor drug efficacy is indicated. We, therefore, propose novel guidelines to support future monitoring programs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. - PubMed
-
- WHO. Deworming for health and development. 2005. Report of the third global meeting of the Partners for Parasitic Control. WHO/CDS/CPE/PVC/2005.14, World Health Organization, Geneva.
-
- Albonico M, Engels D, Savioli L. Monitoring drug efficacy and early detection of drug resistance in human soil-transmitted nematodes: a pressing public health agenda for helminth control. Int J Parasitol. 2004;34:1205–1210. - PubMed
-
- Geerts S, Gryseels B. Anthelmintic resistance in human helminths: a review. Trop Med Int Health. 2001;6:915–921. - PubMed
-
- De Clercq D, Sacko M, Behnke JM, Gilbert F, Dorny P, et al. Failure of mebendazole in treatment of human hookworm infections in the Southern Region of Mali. Am J Trop Med Hyg. 1997;57:25–30. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

