Use of a soluble epoxide hydrolase inhibitor in smoke-induced chronic obstructive pulmonary disease
- PMID: 22180869
- PMCID: PMC3359909
- DOI: 10.1165/rcmb.2011-0359OC
Use of a soluble epoxide hydrolase inhibitor in smoke-induced chronic obstructive pulmonary disease
Abstract
Tobacco smoke-induced chronic obstructive pulmonary disease (COPD) is a prolonged inflammatory condition of the lungs characterized by progressive and largely irreversible airflow limitation attributable to a number of pathologic mechanisms, including bronchitis, bronchiolitis, emphysema, mucus plugging, pulmonary hypertension, and small-airway obstruction. Soluble epoxide hydrolase inhibitors (sEHIs) demonstrated anti-inflammatory properties in a rat model after acute exposure to tobacco smoke. We compared the efficacy of sEHI t-TUCB (trans-4-{4-[3-(4-trifluoromethoxy-phenyl)-ureido]-cyclohexyloxy}-benzoic acid) and the phosphodiesterase-4 (PDE4) inhibitor Rolipram (Biomol International, Enzo Life Sciences, Farmingdale, NY) to reduce lung injury and inflammation after subacute exposure to tobacco smoke over a period of 4 weeks. Pulmonary physiology, bronchoalveolar lavage, cytokine production, and histopathology were analyzed to determine the efficacy of sEHI and Rolipram to ameliorate tobacco smoke-induced inflammation and injury in the spontaneously hypertensive rat. Both t-TUCB and Rolipram inhibited neutrophil elevation in bronchoalveolar lavage. sEHI t-TUCB suppressed IFN-γ, while improving lung function by reducing tobacco smoke-induced total respiratory resistance and tissue damping (small-airway and peripheral tissue resistance). Increases in tobacco smoke-induced alveolar airspace size were attenuated by t-TUCB. Rolipram inhibited the production of airway mucus. Both t-TUCB and Rolipram inhibited vascular remodeling-related growth factor. These findings suggest that sEHI t-TUCB has therapeutic potential for treating COPD by improving lung function and attenuating the lung inflammation and emphysematous changes caused by tobacco smoke. To the best of our knowledge, this is the first report to demonstrate that sEHI exerts significant protective effects after repeated, subacute tobacco smoke-induced lung injury in a rat model of COPD.
Figures
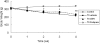

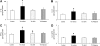



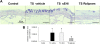

Similar articles
-
Soluble Epoxide Hydrolase Inhibition by t-TUCB Promotes Brown Adipogenesis and Reduces Serum Triglycerides in Diet-Induced Obesity.Int J Mol Sci. 2020 Sep 24;21(19):7039. doi: 10.3390/ijms21197039. Int J Mol Sci. 2020. PMID: 32987880 Free PMC article.
-
Impact of diabetes on male sexual function in streptozotocin-induced diabetic rats: Protective role of soluble epoxide hydrolase inhibitor.Biomed Pharmacother. 2019 Jul;115:108897. doi: 10.1016/j.biopha.2019.108897. Epub 2019 May 15. Biomed Pharmacother. 2019. PMID: 31102913 Free PMC article.
-
Soluble epoxide hydrolase inhibitor, t-TUCB, protects against myocardial ischaemic injury in rats.J Pharm Pharmacol. 2014 Sep;66(9):1251-8. doi: 10.1111/jphp.12251. Epub 2014 Apr 2. J Pharm Pharmacol. 2014. PMID: 24697323 Free PMC article.
-
Club Cell Protein 16 (CC16) Augmentation: A Potential Disease-modifying Approach for Chronic Obstructive Pulmonary Disease (COPD).Expert Opin Ther Targets. 2016 Jul;20(7):869-83. doi: 10.1517/14728222.2016.1139084. Epub 2016 Feb 11. Expert Opin Ther Targets. 2016. PMID: 26781659 Free PMC article. Review.
-
Animal models and mechanisms of tobacco smoke-induced chronic obstructive pulmonary disease (COPD).J Toxicol Environ Health B Crit Rev. 2023 Jul 4;26(5):275-305. doi: 10.1080/10937404.2023.2208886. Epub 2023 May 14. J Toxicol Environ Health B Crit Rev. 2023. PMID: 37183431 Free PMC article. Review.
Cited by
-
Structure-guided discovery of potent and oral soluble epoxide hydrolase inhibitors for the treatment of neuropathic pain.Acta Pharm Sin B. 2022 Mar;12(3):1377-1389. doi: 10.1016/j.apsb.2021.09.018. Epub 2021 Sep 22. Acta Pharm Sin B. 2022. PMID: 35530144 Free PMC article.
-
Vascular Lipidomic Profiling of Potential Endogenous Fatty Acid PPAR Ligands Reveals the Coronary Artery as Major Producer of CYP450-Derived Epoxy Fatty Acids.Cells. 2020 Apr 29;9(5):1096. doi: 10.3390/cells9051096. Cells. 2020. PMID: 32365470 Free PMC article.
-
Identification and optimization of soluble epoxide hydrolase inhibitors with dual potency towards fatty acid amide hydrolase.Bioorg Med Chem Lett. 2018 Feb 15;28(4):762-768. doi: 10.1016/j.bmcl.2018.01.003. Epub 2018 Jan 4. Bioorg Med Chem Lett. 2018. PMID: 29366648 Free PMC article.
-
Positioning new pharmacotherapies for COPD.Int J Chron Obstruct Pulmon Dis. 2015 Jul 24;10:1427-42. doi: 10.2147/COPD.S83758. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 26244017 Free PMC article. Review.
-
Effects of chronic secondhand smoke exposure on cardiovascular regulation and the role of soluble epoxide hydrolase in mice.Front Physiol. 2023 Jun 8;14:1185744. doi: 10.3389/fphys.2023.1185744. eCollection 2023. Front Physiol. 2023. PMID: 37362438 Free PMC article.
References
-
- Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, Mendez RA, Pinto Plata V, Cabral HJ. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004;350:1005–1012 - PubMed
-
- Fox JC, Fitzgerald MF. The role of animal models in the pharmacological evaluation of emerging anti-inflammatory agents for the treatment of COPD. Curr Opin Pharmacol 2009;9:231–242 - PubMed
-
- Kubo S, Kobayashi M, Iwata M, Takahashi K, Miyata K, Shimizu Y. Disease-modifying effect of ASP3258, a novel phosphodiesterase Type 4 inhibitor, on subchronic cigarette smoke exposure–induced lung injury in guinea pigs. Eur J Pharmacol 2011;659:79–84 - PubMed
-
- Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care 2001;46:798–825 - PubMed
-
- Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J 2003;22:672–688 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

