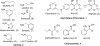Expedient synthesis of a 72-membered isoxazolino-β-ketoamide library by a 2·3-component reaction
- PMID: 22181856
- PMCID: PMC3278521
- DOI: 10.1021/co200199h
Expedient synthesis of a 72-membered isoxazolino-β-ketoamide library by a 2·3-component reaction
Abstract
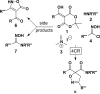
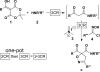
An efficient 2·3-component reaction (2·3CR; a 2-component reaction followed, in one pot, by a3-component reaction) is presented for the synthesis of isoxazolino-β-ketoamides. This 2·3CR proceeds by (i) a Meldrum's acid-generated acyl ketene, which is trapped by an amine to form a β-ketoamide intermediate in a 2CR followed, in one pot, by (ii) a Mannich reaction followed by elimination of dimethyl amine·HCl to generate an α,β-unsaturated β-ketoamide dipolarophile that reacts in a nitrile oxide 1,3-dipolar cycloaddition reaction. This one-pot 2·3CR process delivers the targeted isoxazolino-β-ketoamide product. A total of 72 compounds are presented, all of which have been submitted to the NIH Molecular Libraries Small Molecule Repository for high-throughput biological screening.
Figures
Similar articles
-
Synthesis of isoxazolo[5,4-b]pyridines by microwave-assisted multi-component reactions in water.J Comb Chem. 2009 May-Jun;11(3):428-32. doi: 10.1021/cc800212v. J Comb Chem. 2009. PMID: 19364093
-
Solution-phase synthesis of a diverse library of benzisoxazoles utilizing the [3 + 2] cycloaddition of in situ-generated nitrile oxides and arynes.ACS Comb Sci. 2013 Apr 8;15(4):193-201. doi: 10.1021/co300159g. Epub 2013 Mar 8. ACS Comb Sci. 2013. PMID: 23472819 Free PMC article.
-
Construction of a bicyclic beta-benzyloxy and beta-hydroxy amide library through a multicomponent cyclization reaction.J Comb Chem. 2009 Jul-Aug;11(4):640-4. doi: 10.1021/cc800200h. J Comb Chem. 2009. PMID: 19505108 Free PMC article.
-
Liquid-phase combinatorial library synthesis: recent advances and future perspectives.Comb Chem High Throughput Screen. 2014;17(5):417-38. doi: 10.2174/1386207316666131117172549. Comb Chem High Throughput Screen. 2014. PMID: 24237348 Review.
-
Organic selenium resin in solid phase synthesis and its application in constructing medicinally relevant small organic molecules.Mini Rev Med Chem. 2013 May 1;13(6):854-69. doi: 10.2174/1389557511313060008. Mini Rev Med Chem. 2013. PMID: 23544465 Review.
Cited by
-
An efficient method for the construction of polysubstituted 4-pyridones via self-condensation of β-keto amides mediated by P2O5 and catalyzed by zinc bromide.Beilstein J Org Chem. 2013 Nov 28;9:2681-7. doi: 10.3762/bjoc.9.304. eCollection 2013. Beilstein J Org Chem. 2013. PMID: 24367433 Free PMC article.
References
-
- Trost BM. Atom economy – a challenge for organic synthesis: homogeneous catalysis leads the way. Angew. Chem., Int. Ed. 1995;34:259–281.
- Noyori R. synthesizing our future. Nat. Chem. 2009;1:5–6. - PubMed
- Bienayme H, Hulme C, Oddon G, Schmitt P. Maximizing synthetic efficiency: multi-component rransformations lead the way. Chem. Eur. J. 2000;6:3321–3329. - PubMed
- Posner GH. Multicomponent one-pot annulations forming 3 to 6 bonds. Chem. Rev. 1986;86:831–844.
- Boger DL, Desharnais J, Capps K. Solution-phase combinatorial libraries: modulating cellular signaling by targeting protein-protein or protein-DNA Interactions. Angew. Chem., Int. Ed. 2003;42:4138–4176. - PubMed
-
- Biginelli P. The urea-aldehyde derivatives of acetoacetic esters. Ber. 1891;24:2962–2967.
- Biginelli P. The urea-aldehyde derivatives of acetoacetic esters. Gazz. Chim. Ital. 1893;23:360–416.
-
- Passerni M. Isonitriles. II. Compounds with aldehydes or with ketones and monobasic organic acids. Gazz. Chim. Ital. 1921;51:181–189.
-
- Hantzch A. Synthesis of pyridine derivatives from acetoacetic esters and aldehydeammonia. Liebigs Ann. Chem. 1882;215:1–82.
-
- Ugi I, Meyr R, Fetzer U, Steinbruckner C. Studies on isonitriles. Angew. Chem. 1959;71:386.
- Ugi I, Steinbruckner C. Concerning a new condensation principle. Angew. Chem. 1960;72:267–268.
- Ugi I. The α-addition of immonium ions and anions to isonitriles coupled with secondary reactions. Angew. Chem. 1962;74:9–22.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources