Kalirin-7 is necessary for normal NMDA receptor-dependent synaptic plasticity
- PMID: 22182308
- PMCID: PMC3261125
- DOI: 10.1186/1471-2202-12-126
Kalirin-7 is necessary for normal NMDA receptor-dependent synaptic plasticity
Abstract
Background: Dendritic spines represent the postsynaptic component of the vast majority of excitatory synapses present in the mammalian forebrain. The ability of spines to rapidly alter their shape, size, number and receptor content in response to stimulation is considered to be of paramount importance during the development of synaptic plasticity. Indeed, long-term potentiation (LTP), widely believed to be a cellular correlate of learning and memory, has been repeatedly shown to induce both spine enlargement and the formation of new dendritic spines. In our studies, we focus on Kalirin-7 (Kal7), a Rho GDP/GTP exchange factor (Rho-GEF) localized to the postsynaptic density that plays a crucial role in the development and maintenance of dendritic spines both in vitro and in vivo. Previous studies have shown that mice lacking Kal7 (Kal7(KO)) have decreased dendritic spine density in the hippocampus as well as focal hippocampal-dependent learning impairments.
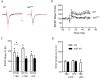
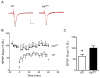
Results: We have performed a detailed electrophysiological characterization of the role of Kal7 in hippocampal synaptic plasticity. We show that loss of Kal7 results in impaired NMDA receptor-dependent LTP and long-term depression, whereas a NMDA receptor-independent form of LTP is shown to be normal in the absence of Kal7.
Conclusions: These results indicate that Kal7 is an essential and selective modulator of NMDA receptor-dependent synaptic plasticity in the hippocampus.
Figures



Similar articles
-
Kalirin binds the NR2B subunit of the NMDA receptor, altering its synaptic localization and function.J Neurosci. 2011 Aug 31;31(35):12554-65. doi: 10.1523/JNEUROSCI.3143-11.2011. J Neurosci. 2011. PMID: 21880917 Free PMC article.
-
Kalirin-7 is an essential component of both shaft and spine excitatory synapses in hippocampal interneurons.J Neurosci. 2008 Jan 16;28(3):711-24. doi: 10.1523/JNEUROSCI.5283-07.2008. J Neurosci. 2008. PMID: 18199770 Free PMC article.
-
Kalirin-7 is required for synaptic structure and function.J Neurosci. 2008 Nov 19;28(47):12368-82. doi: 10.1523/JNEUROSCI.4269-08.2008. J Neurosci. 2008. PMID: 19020030 Free PMC article.
-
Kalirin-7 is a key player in the formation of excitatory synapses in hippocampal neurons.ScientificWorldJournal. 2010 Aug 17;10:1655-66. doi: 10.1100/tsw.2010.148. ScientificWorldJournal. 2010. PMID: 20730383 Free PMC article. Review.
-
Hippocampal long-term synaptic plasticity and signal amplification of NMDA receptors.Crit Rev Neurobiol. 2006;18(1-2):71-84. doi: 10.1615/critrevneurobiol.v18.i1-2.80. Crit Rev Neurobiol. 2006. PMID: 17725510 Review.
Cited by
-
Kalirin as a Novel Treatment Target for Cognitive Dysfunction in Schizophrenia.CNS Drugs. 2022 Jan;36(1):1-16. doi: 10.1007/s40263-021-00884-z. Epub 2021 Dec 20. CNS Drugs. 2022. PMID: 34928485 Review.
-
Targeting the Small GTPase Superfamily through Their Regulatory Proteins.Angew Chem Int Ed Engl. 2020 Apr 16;59(16):6342-6366. doi: 10.1002/anie.201900585. Epub 2020 Jan 30. Angew Chem Int Ed Engl. 2020. PMID: 30869179 Free PMC article. Review.
-
Elimination of Kalrn expression in POMC cells reduces anxiety-like behavior and contextual fear learning.Horm Behav. 2014 Jul;66(2):430-8. doi: 10.1016/j.yhbeh.2014.07.001. Epub 2014 Jul 9. Horm Behav. 2014. PMID: 25014196 Free PMC article.
-
Using Kalirin conditional knockout mice to distinguish its role in dopamine receptor mediated behaviors.BMC Neurosci. 2017 May 23;18(1):45. doi: 10.1186/s12868-017-0363-2. BMC Neurosci. 2017. PMID: 28535798 Free PMC article.
-
Nonenzymatic domains of Kalirin7 contribute to spine morphogenesis through interactions with phosphoinositides and Abl.Mol Biol Cell. 2014 May;25(9):1458-71. doi: 10.1091/mbc.E13-04-0215. Epub 2014 Mar 5. Mol Biol Cell. 2014. PMID: 24600045 Free PMC article.
References
-
- Segal M. Dendritic spines and long-term plasticity. Nature reviews Neuroscience. 2005;6:277–284. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

