Tolerability of NGX-4010, a capsaicin 8% dermal patch, following pretreatment with lidocaine 2.5%/prilocaine 2.5% cream in patients with post-herpetic neuralgia
- PMID: 22182397
- PMCID: PMC3292968
- DOI: 10.1186/1471-2253-11-25
Tolerability of NGX-4010, a capsaicin 8% dermal patch, following pretreatment with lidocaine 2.5%/prilocaine 2.5% cream in patients with post-herpetic neuralgia
Abstract
Background: Post-herpetic neuralgia (PHN) is a common type of neuropathic pain that can severely affect quality of life. NGX-4010, a capsaicin 8% dermal patch, is a localized treatment that can provide patients with significant pain relief for up to 3 months following a single 60-minute application. The NGX-4010 application can be associated with application-site pain and in previous clinical trials pretreatment with a topical 4% lidocaine anesthetic was used to enhance tolerability. The aim of the current investigation was to evaluate tolerability of NGX-4010 after pretreatment with lidocaine 2.5%/prilocaine 2.5% anesthetic cream.
Methods: Twenty-four patients with PHN were pretreated with lidocaine 2.5%/prilocaine 2.5% cream for 60 minutes before receiving a single 60-minute application of NGX-4010. Tolerability was assessed by measuring patch application duration, the proportion of patients completing over 90% of the intended treatment duration, application site-related pain using the Numeric Pain Rating Scale (NPRS), and analgesic medication use to relieve such pain. Safety was assessed by monitoring adverse events (AEs) and dermal irritation using dermal assessment scores.
Results: The mean treatment duration of NGX-4010 was 60.2 minutes and all patients completed over 90% of the intended patch application duration. Pain during application was transient. A maximum mean change in NPRS score of +3.0 was observed at 55 minutes post-patch application; pain scores gradually declined to near pre-anesthetic levels (+0.71) within 85 minutes of patch removal. Half of the patients received analgesic medication on the day of treatment; by Day 7, no patients required medication. The most common AEs were application site-related pain, erythema, edema, and pruritus. All patients experienced mild dermal irritation 5 minutes after patch removal, which subsequently decreased; at Day 7, no irritation was evident. The maximum recorded dermal assessment score was 2.
Conclusion: NGX-4010 was well tolerated following pretreatment with lidocaine 2.5%/prilocaine 2.5% cream in patients with PHN. The tolerability of the patch application appeared comparable with that seen in other studies that used 4% lidocaine cream as the pretreatment anesthetic. This study is registered at http://www.clinicaltrials.gov as number NCT00916942.
Figures


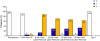
References
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

