Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury
- PMID: 22182453
- PMCID: PMC3980444
- DOI: 10.1016/j.canlet.2011.12.012
Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury
Abstract
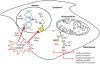
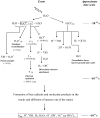
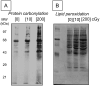
Cellular exposure to ionizing radiation leads to oxidizing events that alter atomic structure through direct interactions of radiation with target macromolecules or via products of water radiolysis. Further, the oxidative damage may spread from the targeted to neighboring, non-targeted bystander cells through redox-modulated intercellular communication mechanisms. To cope with the induced stress and the changes in the redox environment, organisms elicit transient responses at the molecular, cellular and tissue levels to counteract toxic effects of radiation. Metabolic pathways are induced during and shortly after the exposure. Depending on radiation dose, dose-rate and quality, these protective mechanisms may or may not be sufficient to cope with the stress. When the harmful effects exceed those of homeostatic biochemical processes, induced biological changes persist and may be propagated to progeny cells. Physiological levels of reactive oxygen and nitrogen species play critical roles in many cellular functions. In irradiated cells, levels of these reactive species may be increased due to perturbations in oxidative metabolism and chronic inflammatory responses, thereby contributing to the long-term effects of exposure to ionizing radiation on genomic stability. Here, in addition to immediate biological effects of water radiolysis on DNA damage, we also discuss the role of mitochondria in the delayed outcomes of ionization radiation. Defects in mitochondrial functions lead to accelerated aging and numerous pathological conditions. Different types of radiation vary in their linear energy transfer (LET) properties, and we discuss their effects on various aspects of mitochondrial physiology. These include short and long-term in vitro and in vivo effects on mitochondrial DNA, mitochondrial protein import and metabolic and antioxidant enzymes.
Copyright © 2012 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Figures


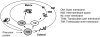

References
-
- Hall EJ, Giaccia AJ. Radiobiology for the Radiologist. 6th. Lippincott Williams & Wilkins; Philadelphia, PA: 2006.
-
- Spitz DR, Azzam EI, Li JJ, Gius D. Metabolic oxidation/reduction reactions and cellular responses to ionizing radiation: a unifying concept in stress response biology. Cancer Metastasis Rev. 2004;23:311–322. - PubMed
-
- Kryston TB, Georgiev AB, Pissis P, Georgakilas AG. Role of oxidative stress and DNA damage in human carcinogenesis. Mutat Res. 2011;711:193–201. - PubMed
-
- Tamminga J, Kovalchuk O. Role of DNA damage and epigenetic DNA methylation changes in radiation-induced genomic instability and bystander effects in germline in vivo. Current molecular pharmacology. 2011;4:115–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

