Chronic pulsatile hyperglycemia reduces insulin secretion and increases accumulation of reactive oxygen species in fetal sheep islets
- PMID: 22182602
- PMCID: PMC3516619
- DOI: 10.1530/JOE-11-0300
Chronic pulsatile hyperglycemia reduces insulin secretion and increases accumulation of reactive oxygen species in fetal sheep islets
Abstract
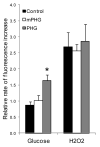
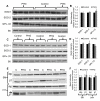
Children from diabetic pregnancies have a greater incidence of type 2 diabetes. Our objective was to determine if exposure to mild-moderate hyperglycemia, by modeling managed diabetic pregnancies, affects fetal β-cell function. In sheep fetuses, β-cell responsiveness was examined after 2 weeks of sustained hyperglycemia with 3 pulses/day, mimicking postprandial excursions, and compared to saline-infused controls (n = 10). Two pulsatile hyperglycemia (PHG) treatments were studied: mild (mPHG, n = 5) with +15% sustained and +55% pulse; and moderate (PHG, n = 10) with +20% sustained and +100% pulse. Fetal glucose-stimulated insulin secretion and glucose-potentiated arginine insulin secretion were lower (P < 0.05) in PHG (0.86 ± 0.13 and 2.91 ± 0.39 ng/ml plasma insulin) but not in mPHG fetuses (1.21 ± 0.08 and 4.25 ± 0.56 ng/ml) compared to controls (1.58 ± 0.25 and 4.51 ± 0.56 ng/ml). Islet insulin content was 35% lower in PHG and 35% higher in mPHG vs controls (P < 0.01). Insulin secretion and maximally stimulated insulin release were also reduced (P < 0.05) in PHG islets due to lower islet insulin content. Isolated PHG islets also had 63% greater (P < 0.01) reactive oxygen species (ROS) accumulation at 11.1 mmol/l glucose than controls (P < 0.01), but oxidative damage was not detected in islet proteins. PHG fetuses showed evidence of oxidative damage to skeletal muscle proteins (P < 0.05) but not insulin resistance. Our findings show that PHG induced dysregulation of islet ROS handling and decreased islet insulin content, but these outcomes are independent. The β-cell outcomes were dependent on the severity of hyperglycemia because mPHG fetuses had no distinguishable impairments in ROS handling or insulin secretion but greater insulin content.
Figures




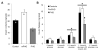

References
-
- Aerts L, Holemans K, Van Assche FA. Maternal diabetes during pregnancy: consequences for the offspring. Diabetes Metab Rev. 1990;6:147–167. - PubMed
-
- Aerts L, van Assche FA. Rat foetal endocrine pancreas in experimental diabetes. J Endocrinol. 1977;73:339–346. - PubMed
-
- Aerts L, Van Assche FA. Endocrine pancreas in the offspring of rats with experimentally induced diabetes. J Endocrinol. 1981;88:81–88. - PubMed
-
- Aerts L, Vercruysse L, Van Assche FA. The endocrine pancreas in virgin and pregnant offspring of diabetic pregnant rats. Diabetes Res Clin Pract. 1997;38:9–19. - PubMed
-
- American Diabetes Association Gestational Diabetes Mellitus. Diabetes Care. 2004;27:S88–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

