Evaluation of the safety and efficacy of MonoMax® suture material for abdominal wall closure after primary midline laparotomy-a controlled prospective multicentre trial: ISSAAC [NCT005725079]
- PMID: 22183105
- PMCID: PMC3281202
- DOI: 10.1007/s00423-011-0884-6
Evaluation of the safety and efficacy of MonoMax® suture material for abdominal wall closure after primary midline laparotomy-a controlled prospective multicentre trial: ISSAAC [NCT005725079]
Abstract
Purpose: Different suture techniques and various suture materials are in use to close midline incisions after primary laparotomy. The ISSAAC study aimed to assess the safety and efficacy of the new ultra-long-term absorbable, elastic monofilament suture material MonoMax® for abdominal wall closure.
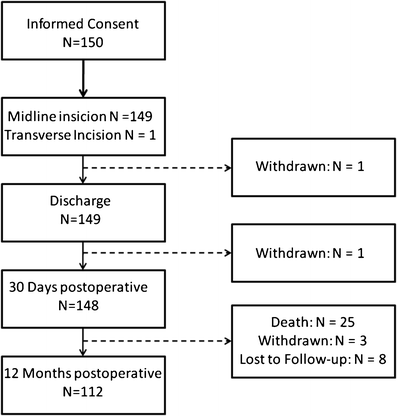
Methods: This is a single-arm, multicentre prospective study that included 150 patients undergoing a primary elective midline incision. The control group consists of 141 patients from the INSECT study who received MonoPlus® or PDS® for abdominal wall closure. The incidences of burst abdomen and wound infection until the day of discharge were defined as the primary composite endpoints. The rate of incisional hernias 1 year after surgery, the length of postoperative hospital stay and safety parameters served as secondary endpoints. The study has been registered under www.clinicaltrials.gov [NCT005725079].
Results: Eleven patients in the ISSAAC study [7.3%; 95% CI = (3.9; 13.1%)] experienced wound infection or burst abdomen until the day of discharge as compared to 16 [11.3%; 95% CI = (6.6; 17.8%)] patients in the INSECT control group (p = 0.31). The length of postoperative hospital stay was comparable in both study groups. One year after surgery, incisional hernias were observed in 21 ISSAAC patients (14.0%) in contrast to 30 hernias (21.3%) in the INSECT control group.
Conclusions: The ultra-long-term absorbable, elastic monofilament suture material MonoMax® is safe and efficient for abdominal wall closure.
Figures
Similar articles
-
A historically controlled, single-arm, multi-centre, prospective trial to evaluate the safety and efficacy of MonoMax suture material for abdominal wall closure after primary midline laparotomy. ISSAAC-Trial [NCT005725079].BMC Surg. 2008 Jul 21;8:12. doi: 10.1186/1471-2482-8-12. BMC Surg. 2008. PMID: 18644124 Free PMC article. Clinical Trial.
-
PREBIOUS trial: a multicenter randomized controlled trial of PREventive midline laparotomy closure with a BIOabsorbable mesh for the prevention of incisional hernia: rationale and design.Contemp Clin Trials. 2014 Nov;39(2):335-41. doi: 10.1016/j.cct.2014.10.009. Epub 2014 Nov 1. Contemp Clin Trials. 2014. PMID: 25445313 Clinical Trial.
-
Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541).Ann Surg. 2009 Apr;249(4):576-82. doi: 10.1097/SLA.0b013e31819ec6c8. Ann Surg. 2009. PMID: 19300233 Clinical Trial.
-
Abdominal incision defect following AAA-surgery (AIDA): 2-year results of prophylactic onlay-mesh augmentation in a multicentre, double-blind, randomised controlled trial.Updates Surg. 2022 Jun;74(3):1105-1116. doi: 10.1007/s13304-021-01125-0. Epub 2021 Jul 21. Updates Surg. 2022. PMID: 34287760 Free PMC article. Review.
-
[Current standards of abdominal wall closure techniques : Conventional suture techniques].Chirurg. 2016 Sep;87(9):737-743. doi: 10.1007/s00104-016-0231-0. Chirurg. 2016. PMID: 27392763 Review. German.
Cited by
-
Delayed closure of open abdomen in septic patients is facilitated by combined negative pressure wound therapy and dynamic fascial suture.Surg Endosc. 2014 Mar;28(3):735-40. doi: 10.1007/s00464-013-3251-6. Epub 2013 Oct 23. Surg Endosc. 2014. PMID: 24149855 Clinical Trial.
-
PRevention of INCisional hernia after liver transplantation (PRINC trial): study protocol for a randomized controlled trial.Trials. 2019 Jun 20;20(1):371. doi: 10.1186/s13063-019-3477-2. Trials. 2019. PMID: 31221206 Free PMC article.
-
Effect of suture technique on the occurrence of incisional hernia after elective midline abdominal wall closure: study protocol for a randomized controlled trial.Trials. 2015 Feb 15;16:52. doi: 10.1186/s13063-015-0572-x. Trials. 2015. PMID: 25887884 Free PMC article. Clinical Trial.
-
Small bites versus large bites during fascial closure of midline laparotomies: a systematic review and meta-analysis.Langenbecks Arch Surg. 2024 Mar 22;409(1):104. doi: 10.1007/s00423-024-03293-0. Langenbecks Arch Surg. 2024. PMID: 38519824
-
Three-year follow-up analysis of the short-stitch versus long-stitch technique for elective midline abdominal closure randomized-controlled (ESTOIH) trial.Hernia. 2024 Aug;28(4):1283-1291. doi: 10.1007/s10029-024-03025-9. Epub 2024 Mar 27. Hernia. 2024. PMID: 38536592 Free PMC article. Clinical Trial.
References
-
- Seiler CM, Bruckner T, Diener MK, Papyan A, Golcher H, Seidlmayer C, Franck A, Kieser M, Buchler MW, Knaebel HP. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541) Ann Surg. 2009;249(4):576–582. doi: 10.1097/SLA.0b013e31819ec6c8. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous