Pharmacokinetics of lamivudine and lamivudine-triphosphate after administration of 300 milligrams and 150 milligrams once daily to healthy volunteers: results of the ENCORE 2 study
- PMID: 22183172
- PMCID: PMC3294959
- DOI: 10.1128/AAC.05599-11
Pharmacokinetics of lamivudine and lamivudine-triphosphate after administration of 300 milligrams and 150 milligrams once daily to healthy volunteers: results of the ENCORE 2 study
Abstract
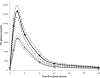
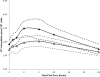
There is interest in evaluating the efficacy of lower doses of certain antiretrovirals for clinical care. We determined here the bioequivalence of plasma lamivudine (3TC) and intracellular 3TC-triphosphate (3TC-TP) concentrations after the administration of two different doses. ENCORE 2 was a randomized crossover study. Subjects received 3TC at 300 and 150 mg once daily for 10 days (arm 1; n = 13) or vice versa (arm 2; n = 11), separated by a 10-day washout. Pharmacokinetic (PK) profiles (0 to 24 h) were assessed on days 10 and 30. Plasma 3TC and 3TC-TP levels in peripheral blood mononuclear cells were quantified by high-performance liquid chromatography-tandem mass spectrometry. Within-subject changes in PK parameters (the area under the concentration-time curve from 0 to 24 h [AUC(0-24)], the trough concentration of drug in plasma at 24 h [C(24)], and the maximum concentration of drug in plasma [C(max)]) were evaluated by determining the geometric mean ratios (GMRs) adjusted for study arm, period, and intra-individual variation. Regimens were considered bioequivalent if the 90% confidence interval (90% CI) fell within the range of 0.8 to 1.25. A total of 24 subjects completed the study. The GM (90% CI) 3TC AUC(0-24)), expressed as ng·h/ml, for the 300- and 150-mg doses were 8,354 (7,609 to 9,172) and 4,773 (4,408 to 5,169), respectively. Bioequivalence in 3TC PK following the administration of 300 and 150 mg was not demonstrated: the GMRs for AUC(0-24), C(24), and C(max) were 0.57 (0.55 to 0.60), 0.63 (0.59 to 0.67), and 0.56 (0.53 to 0.60), respectively. The GM (90% CI) 3TC-TP AUC(0-24) values (pmol·h/10(6) cells) for the 300- and 150-mg doses were 59.5 (51.8 to 68.3) and 44.0 (38.0 to 51.0), respectively. Bioequivalence in 3TC-TP PK following the administration of 300 and 150 mg was not demonstrated: the GMRs for AUC(0-24), C(24), and C(max) were 0.73 (0.64 to 0.83), 0.82 (0.68 to 0.99), and 0.70 (0.61 to 0.82), respectively. We found that 3TC at 150 mg is not bioequivalent to the standard regimen of 300 mg, indicating that saturation of cytosine phosphorylation pathways is not achieved at a dose of 150 mg.
Figures
Similar articles
-
Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily.Antimicrob Agents Chemother. 2004 Jan;48(1):176-82. doi: 10.1128/AAC.48.1.176-182.2004. Antimicrob Agents Chemother. 2004. PMID: 14693537 Free PMC article. Clinical Trial.
-
Pharmacokinetic Modeling of Lamivudine and Zidovudine Triphosphates Predicts Differential Pharmacokinetics in Seminal Mononuclear Cells and Peripheral Blood Mononuclear Cells.Antimicrob Agents Chemother. 2015 Oct;59(10):6395-401. doi: 10.1128/AAC.01148-15. Epub 2015 Aug 3. Antimicrob Agents Chemother. 2015. PMID: 26239974 Free PMC article.
-
Intracellular nucleoside triphosphate concentrations in HIV-infected patients on dual nucleoside reverse transcriptase inhibitor therapy.Antivir Ther. 2007;12(6):981-6. Antivir Ther. 2007. PMID: 17926654
-
Joint population pharmacokinetic analysis of zidovudine, lamivudine, and their active intracellular metabolites in HIV patients.Antimicrob Agents Chemother. 2011 Jul;55(7):3423-31. doi: 10.1128/AAC.01487-10. Epub 2011 May 16. Antimicrob Agents Chemother. 2011. PMID: 21576446 Free PMC article.
-
Perspectives on the molecular mechanism of inhibition and toxicity of nucleoside analogs that target HIV-1 reverse transcriptase.Biochim Biophys Acta. 2002 Jul 18;1587(2-3):296-9. doi: 10.1016/s0925-4439(02)00092-3. Biochim Biophys Acta. 2002. PMID: 12084471 Review.
Cited by
-
Evaluation of the Activity of Lamivudine and Zidovudine against Ebola Virus.PLoS One. 2016 Nov 30;11(11):e0166318. doi: 10.1371/journal.pone.0166318. eCollection 2016. PLoS One. 2016. PMID: 27902714 Free PMC article.
-
A novel formulation enabled transformation of 3-HIV drugs tenofovir-lamivudine-dolutegravir from short-acting to long-acting all-in-one injectable.AIDS. 2023 Nov 15;37(14):2131-2136. doi: 10.1097/QAD.0000000000003706. Epub 2023 Aug 24. AIDS. 2023. PMID: 37650755 Free PMC article.
-
Viral suppression following switch to second-line antiretroviral therapy: associations with nucleoside reverse transcriptase inhibitor resistance and subtherapeutic drug concentrations prior to switch.J Infect Dis. 2014 Mar 1;209(5):711-20. doi: 10.1093/infdis/jit411. Epub 2013 Aug 13. J Infect Dis. 2014. PMID: 23943851 Free PMC article.
-
Nucleotide Reverse Transcriptase Inhibitors: A Thorough Review, Present Status and Future Perspective as HIV Therapeutics.Curr HIV Res. 2017;15(6):411-421. doi: 10.2174/1570162X15666171120110145. Curr HIV Res. 2017. PMID: 29165087 Free PMC article. Review.
-
Lamivudine/Tenofovir Disoproxil Fumarate is an Appropriate PrEP Regimen.Drugs. 2020 Dec;80(18):1881-1888. doi: 10.1007/s40265-020-01419-4. Drugs. 2020. PMID: 33040323 Free PMC article. Review.
References
-
- Anderson PL, Kakuda TN, Kawle S, Fletcher CV. 2003. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS 17:2159–2168 - PubMed
-
- Anderson PL, et al. 2011. The cellular pharamcology of zidovudine and lamivudine according to HIV-status and gender, abstr. O_6 Abstr. 12th Int Workshop Clin. Pharmacol. HIV Ther
-
- Anderson PL, et al. 2007. Concentrations of zidovudine- and lamivudine-triphosphate according to cell type in HIV-seronegative adults. AIDS 21:1849–1854 - PubMed
-
- Bartlett JA, et al. 1996. Lamivudine plus zidovudine compared with zalcitabine plus zidovudine in patients with HIV infection. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 125:161–172 - PubMed
-
- Cammack N, et al. 1992. Cellular metabolism of (−) enantiomeric 2′-deoxy-3′-thiacytidine. Biochem. Pharmacol. 43:2059–2064 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

