Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir
- PMID: 22183173
- PMCID: PMC3294934
- DOI: 10.1128/AAC.05739-11
Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir
Abstract
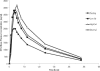
Healthy subjects received dolutegravir at 50 mg in a single-dose crossover study while they were in the fasted state or with low-, moderate-, or high-fat meals. Food increased dolutegravir exposure and reduced the rate of absorption. The area under the concentration-time curve from 0 h to infinity (AUC(0-∞)) increased by 33%, 41%, and 66% when administered with low-, moderate-, or high-fat meals, respectively, compared with fasting. This increase in dolutegravir exposure is not anticipated to impact clinical safety, and therefore dolutegravir can be taken with or without food and without regard to fat content.
Figures
References
-
- Brainard DM, et al. 2011. Effect of low-, moderate-, and high-fat meals on raltegravir pharmacokinetics. J. Clin. Pharmacol. 51:422–477 - PubMed
-
- Bristol-Myers Squibb 2011. Atripla (package insert). Bristol-Myers Squibb and Gilead Sciences, New York, NY
-
- Lenz JC, Rockstroh JK. 2011. S/GSK1349572, a new integrase inhibitor for the treatment of HIV: promises and challenges. Expert Opin. Invest. Drugs 20:537–548 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical