A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P₂-diacylglycerol signalling
- PMID: 22183723
- PMCID: PMC3381819
- DOI: 10.1113/jphysiol.2011.221358
A self-limiting regulation of vasoconstrictor-activated TRPC3/C6/C7 channels coupled to PI(4,5)P₂-diacylglycerol signalling
Abstract
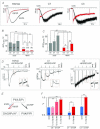
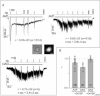
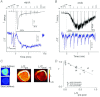
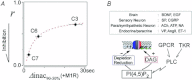
Activation of transient receptor potential (TRP) canonical TRPC3/C6/C7 channels by diacylglycerol (DAG) upon stimulation of phospholipase C (PLC)-coupled receptors results in the breakdown of phosphoinositides (PIPs). The critical importance of PIPs to various ion-transporting molecules is well documented, but their function in relation to TRPC3/C6/C7 channels remains controversial. By using an ectopic voltage-sensing PIP phosphatase (DrVSP), we found that dephosphorylation of PIPs robustly inhibits currents induced by carbachol (CCh), 1-oleolyl-2-acetyl-sn-glycerol (OAG) or RHC80267 in TRPC3, TRPC6 and TRPC7 channels, though the strength of the DrVSP-mediated inhibition (VMI) varied among the channels with a rank order of C7>C6>C3. Pharmacological and molecular interventions suggest that depletion of phosphatidylinositol 4,5-bisphosphate (PI(4,5)P₂) is most likely the critical event for VMI in all three channels.When the PLC catalytic signal was vigorously activated through overexpression of the muscarinic type-I receptor (M1R), the inactivation of macroscopic TRPC currents was greatly accelerated in the same rank order as the VMI, and VMI of these currents was attenuated or lost. VMI was also rarely detected in vasopressin-induced TRPC6-like currents inA7r5 vascular smooth muscle cells, indicating that the inactivation by PI(4,5)P₂ depletion underlies the physiological condition. Simultaneous fluorescence resonance energy transfer (FRET)-based measurement of PI(4,5)P₂ levels and TRPC6 currents confirmed that VMI magnitude reflects the degree of PI(4,5)P₂ depletion. These results demonstrate that TRPC3/C6/C7 channels are differentially regulated by depletion of PI(4,5)P₂, and that the bimodal signal produced by PLC activation controls these channels in a self-limiting manner.
Figures






Similar articles
-
PLC-mediated PI(4,5)P2 hydrolysis regulates activation and inactivation of TRPC6/7 channels.J Gen Physiol. 2014 Feb;143(2):183-201. doi: 10.1085/jgp.201311033. J Gen Physiol. 2014. PMID: 24470487 Free PMC article.
-
Voltage-sensing phosphatase reveals temporal regulation of TRPC3/C6/C7 channels by membrane phosphoinositides.Channels (Austin). 2012 May-Jun;6(3):206-9. doi: 10.4161/chan.20883. Epub 2012 May 1. Channels (Austin). 2012. PMID: 22760061 Free PMC article.
-
Ins(1,4,5)P3 interacts with PIP2 to regulate activation of TRPC6/C7 channels by diacylglycerol in native vascular myocytes.J Physiol. 2010 May 1;588(Pt 9):1419-33. doi: 10.1113/jphysiol.2009.185256. Epub 2010 Mar 8. J Physiol. 2010. PMID: 20211974 Free PMC article.
-
Signalling mechanisms for TRPC3 channels.Novartis Found Symp. 2004;258:123-33; discussion 133-9, 155-9, 263-6. Novartis Found Symp. 2004. PMID: 15104179 Review.
-
Role of phosphoinositol 4,5-bisphosphate and diacylglycerol in regulating native TRPC channel proteins in vascular smooth muscle.Cell Calcium. 2009 Jun;45(6):574-82. doi: 10.1016/j.ceca.2009.02.007. Epub 2009 Mar 25. Cell Calcium. 2009. PMID: 19324408 Review.
Cited by
-
Mechanosensitive cation currents through TRPC6 and Piezo1 channels in human pulmonary arterial endothelial cells.Am J Physiol Cell Physiol. 2022 Oct 1;323(4):C959-C973. doi: 10.1152/ajpcell.00313.2022. Epub 2022 Aug 15. Am J Physiol Cell Physiol. 2022. PMID: 35968892 Free PMC article.
-
Critical contributions of pre-S1 shoulder and distal TRP box in DAG-activated TRPC6 channel by PIP2 regulation.Sci Rep. 2022 Jun 24;12(1):10766. doi: 10.1038/s41598-022-14766-x. Sci Rep. 2022. PMID: 35750783 Free PMC article.
-
Phosphoinositides regulate ion channels.Biochim Biophys Acta. 2015 Jun;1851(6):844-56. doi: 10.1016/j.bbalip.2014.09.010. Epub 2014 Sep 18. Biochim Biophys Acta. 2015. PMID: 25241941 Free PMC article. Review.
-
Ameliorated biomechanical properties of carotid arteries by puerarin in spontaneously hypertensive rats.BMC Complement Med Ther. 2021 Jun 22;21(1):173. doi: 10.1186/s12906-021-03345-8. BMC Complement Med Ther. 2021. PMID: 34154575 Free PMC article.
-
TRPC4 as a coincident detector of Gi/o and Gq/11 signaling: mechanisms and pathophysiological implications.Curr Opin Physiol. 2020 Oct;17:34-41. doi: 10.1016/j.cophys.2020.06.008. Epub 2020 Jul 2. Curr Opin Physiol. 2020. PMID: 32851198 Free PMC article.
References
-
- Ben-Chaim Y, Chanda B, Dascal N, Bezanilla F, Parnas I, Parnas H. Movement of ‘gating charge’ is coupled to ligand binding in a G-protein-coupled receptor. Nature. 2006;444:106–109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

