Inhibition of voltage-gated proton channels by local anaesthetics in GMI-R1 rat microglia
- PMID: 22183729
- PMCID: PMC3381313
- DOI: 10.1113/jphysiol.2011.218149
Inhibition of voltage-gated proton channels by local anaesthetics in GMI-R1 rat microglia
Abstract
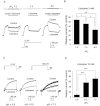
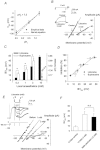
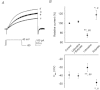
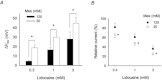
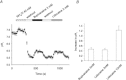
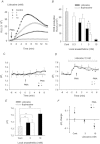
Voltage-gated proton channels play crucial roles during the respiratory burst in phagocytes, such as microglia. As local anaesthetics have a variety of anti-inflammatory properties, including inhibition of phagocytosis, they may act on the proton channels. Most local anaesthetics are tertiary amines and may affect proton channels through modification of pH(i) as weak bases. To test these hypotheses, the effects of lidocaine and bupivacaine on proton channels were examined in a rat microglial cell line (GMI-R1) as a function of pH(o) and pH(i). Both lidocaine and bupivacaine reversibly decreased the current, with IC(50) values of ∼1.2 and ∼0.5 mM, respectively, at pH(o)/pH(i) 7.3/5.5. The inhibition was enhanced with either pH(o) increase or pH(i) decrease, suggesting that the protonation of the base forms inside the cell contributed to the inhibitory effects. Both local anaesthetics shifted the reversal potentials to more positive voltages, indicating increases in pH(i). The potencies of inhibition were correlated well with the degree of increase in pH(i). The lidocaine-induced inhibition was eliminated when the pH(i) increases were cancelled by co-application of a weak acid, butyrate. The cytosolic alkalizations by lidocaine and bupivacaine were confirmed using a pH-sensitive fluorescent dye, BCECF, in non-voltage-clamped cells. Furthermore, chemiluminescence measurement proved that both anaesthetics inhibited production of reactive oxygen species by the cells. In conclusion, lidocaine and bupivacaine inhibit proton channels primarily by the weak base mechanism via an increase in pH(i). This is a novel mechanism underlying actions of local anaesthtics.
Figures



Similar articles
-
The effect of lipid emulsion on intracellular bupivacaine as a mechanism of lipid resuscitation: an electrophysiological study using voltage-gated proton channels.Anesth Analg. 2013 Dec;117(6):1293-301. doi: 10.1213/ANE.0000000000000011. Anesth Analg. 2013. PMID: 24257378
-
Local anaesthetics block hyperpolarization-activated inward current in rat small dorsal root ganglion neurones.Br J Pharmacol. 2003 Aug;139(7):1273-80. doi: 10.1038/sj.bjp.0705363. Br J Pharmacol. 2003. PMID: 12890706 Free PMC article.
-
Temporal fluctuations of voltage-gated proton currents in rat spinal microglia via pH-dependent and -independent mechanisms.Neurosci Res. 2000 Nov;38(3):265-71. doi: 10.1016/s0168-0102(00)00170-x. Neurosci Res. 2000. PMID: 11070193
-
Voltage-gated proton channels in microglia.Prog Neurobiol. 2001 Jun;64(3):277-305. doi: 10.1016/s0301-0082(00)00062-9. Prog Neurobiol. 2001. PMID: 11240310 Review.
-
Pharmacology of voltage-gated proton channels.Curr Pharm Des. 2007;13(23):2400-20. doi: 10.2174/138161207781368675. Curr Pharm Des. 2007. PMID: 17692009 Review.
Cited by
-
Viability and intracellular nitric oxide generation in the umbilical cord blood CD34+CD133- and CD34+CD133+ cell populations exposed to local anaesthetics.Cent Eur J Immunol. 2020;45(4):369-376. doi: 10.5114/ceji.2020.103410. Epub 2021 Jan 30. Cent Eur J Immunol. 2020. PMID: 33658886 Free PMC article.
-
Acid-inducible proton influx currents in the plasma membrane of murine osteoclast-like cells.Pflugers Arch. 2016 May;468(5):837-47. doi: 10.1007/s00424-016-1796-7. Epub 2016 Feb 3. Pflugers Arch. 2016. PMID: 26843093
-
Nicotine inhibits activation of microglial proton currents via interactions with α7 acetylcholine receptors.J Physiol Sci. 2017 Jan;67(1):235-245. doi: 10.1007/s12576-016-0460-5. Epub 2016 Jun 2. J Physiol Sci. 2017. PMID: 27256075 Free PMC article.
-
Pain after knee arthroplasty: an unresolved issue.Knee Surg Sports Traumatol Arthrosc. 2014 Aug;22(8):1744-58. doi: 10.1007/s00167-013-2750-2. Epub 2013 Nov 8. Knee Surg Sports Traumatol Arthrosc. 2014. PMID: 24201900 Review.
-
The Voltage-Gated Proton Channel: A Riddle, Wrapped in a Mystery, inside an Enigma.Biochemistry. 2015 Jun 2;54(21):3250-68. doi: 10.1021/acs.biochem.5b00353. Epub 2015 May 20. Biochemistry. 2015. PMID: 25964989 Free PMC article. Review.
References
-
- Arakawa K, Takahashi H, Nakagawa S, Ogawa S. The effects of lidocaine on superoxide production and p47 Phox translocation in opsonized zymosan-activated neutrophils. Anesth Analg. 2001;93:1501–1506. - PubMed
-
- Cullen BF, Haschke RH. Local anesthetic inhibition of phagocytosis and metabolism of human leukocytes. Anesthesiology. 1974;40:142–146. - PubMed
-
- DeCoursey TE. Voltage-gated proton channels and other proton transfer pathways. Physiol Rev. 2003;83:475–579. - PubMed
-
- Eder C, DeCoursey TE. Voltage-gated proton channels in microglia. Prog Neurobiol. 2001;64:277–305. - PubMed
-
- Eder C, Fischer HG, Hadding U, Heinemann U. Properties of voltage-gated currents of microglia developed using macrophage colony-stimulating factor. Pflugers Arch. 1995;430:526–533. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

