Biotransformation of (1S)-2-carene and (1S)-3-carene by Picea abies suspension culture
- PMID: 22183881
- PMCID: PMC6264755
- DOI: 10.3390/molecules161210541
Biotransformation of (1S)-2-carene and (1S)-3-carene by Picea abies suspension culture
Abstract
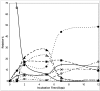
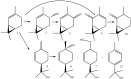
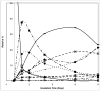
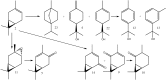
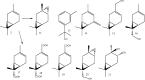
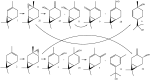
Biotransformation of (1S)-2-carene and (1S)-3-carene by Picea abies suspension culture led to the formation of oxygenated products. (1S)-2-Carene was transformed slowly and the final product was identified as (1S)-2-caren-4-one. On the other hand, the transformation of (1S)-3-carene was rapid and finally led to the formation of (1S)-3-caren-5-one and (1S)-2-caren-4-one as equally abundant major products. The time-course of the reaction indicates that some products abundant at the beginning of the reaction (e.g. (1S, 3S, 4R)-3,4-epoxycarane and (1R)-p-mentha-1(7),2-dien-8-ol) were consumed by a subsequent transformations. Thus, a precise selection of the biotransformation time may be used for a production of specific compounds.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Transformation of terpenes using a Picea abies suspension culture.J Biotechnol. 2004 Jan 22;107(2):173-84. doi: 10.1016/j.jbiotec.2003.10.009. J Biotechnol. 2004. PMID: 14711500
-
Transformation of alpha-pinene using Picea abies suspension culture.J Nat Prod. 2003 Mar;66(3):337-43. doi: 10.1021/np020426m. J Nat Prod. 2003. PMID: 12662089
-
Formation of trans-verbenol and verbenone from alpha-pinene catalysed by immobilised Picea abies cells.Biosci Biotechnol Biochem. 2005 Feb;69(2):321-5. doi: 10.1271/bbb.69.321. Biosci Biotechnol Biochem. 2005. PMID: 15725657
-
Biotransformations of monoterpenes by photoautotrophic micro-organisms.J Appl Microbiol. 2014 Dec;117(6):1523-36. doi: 10.1111/jam.12632. Epub 2014 Sep 26. J Appl Microbiol. 2014. PMID: 25175902 Review.
-
Biotransformation of α- and β-pinene into flavor compounds.Appl Microbiol Biotechnol. 2017 Mar;101(5):1805-1817. doi: 10.1007/s00253-016-8066-7. Epub 2017 Jan 20. Appl Microbiol Biotechnol. 2017. PMID: 28105487 Review.
Cited by
-
Assessment of metabolome diversity in black and white pepper in response to autoclaving using MS- and NMR-based metabolomics and in relation to its remote and direct antimicrobial effects against food-borne pathogens.RSC Adv. 2024 Apr 3;14(15):10799-10813. doi: 10.1039/d4ra00100a. eCollection 2024 Mar 26. RSC Adv. 2024. PMID: 38572341 Free PMC article.
-
Comparison of different extraction methods of Brazilian "pacová" (Renealmia petasites Gagnep.) oilseeds for the determination of lipid and terpene composition, antioxidant capacity, and inhibitory effect on neurodegenerative enzymes.Food Chem X. 2021 Oct 15;12:100140. doi: 10.1016/j.fochx.2021.100140. eCollection 2021 Dec 30. Food Chem X. 2021. PMID: 34746747 Free PMC article.
-
High-frequency direct organogenesis, genetic homogeneity, and root ultra-structural study in Zingiber anamalayanum Sujanapal & Sasidh: a medicinally important wild relative of ginger.3 Biotech. 2025 Jun;15(6):167. doi: 10.1007/s13205-025-04344-z. Epub 2025 May 15. 3 Biotech. 2025. PMID: 40386634
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

