The receptor that tames the innate immune response
- PMID: 22183892
- PMCID: PMC3356428
- DOI: 10.2119/molmed.2011.00414
The receptor that tames the innate immune response
Abstract
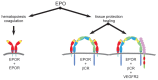
Tissue injury, hypoxia and significant metabolic stress activate innate immune responses driven by tumor necrosis factor (TNF)-α and other proinflammatory cytokines that typically increase damage surrounding a lesion. In a compensatory protective response, erythropoietin (EPO) is synthesized in surrounding tissues, which subsequently triggers antiinflammatory and antiapoptotic processes that delimit injury and promote repair. What we refer to as the sequelae of injury or disease are often the consequences of this intentionally discoordinated, primitive system that uses a "scorched earth" strategy to rid the invader at the expense of a serious lesion. The EPO-mediated tissue-protective system depends on receptor expression that is upregulated by inflammation and hypoxia in a distinctive temporal and spatial pattern. The tissue-protective receptor (TPR) is generally not expressed by normal tissues but becomes functional immediately after injury. In contrast to robust and early receptor expression within the immediate injury site, EPO production is delayed, transient and relatively weak. The functional EPO receptor that attenuates tissue injury is distinct from the hematopoietic receptor responsible for erythropoiesis. On the basis of current evidence, the TPR is composed of the β common receptor subunit (CD131) in combination with the same EPO receptor subunit that is involved in erythropoiesis. Additional receptors, including that for the vascular endothelial growth factor, also appear to be a component of the TPR in some tissues, for example, the endothelium. The discoordination of the EPO response system and its relative weakness provide a window of opportunity to intervene with the exogenous ligand. Recently, molecules were designed that preferentially activate only the TPR and thus avoid the potential adverse consequences of activating the hematopoietic receptor. On administration, these agents successfully substitute for a relative deficiency of EPO production in damaged tissues in multiple animal models of disease and may pave the way to effective treatment of a wide variety of insults that cause tissue injury, leading to profoundly expanded lesions and attendant, irreversible sequelae.
Figures
Similar articles
-
Erythropoietin and its derivatives: from tissue protection to immune regulation.Cell Death Dis. 2020 Feb 3;11(2):79. doi: 10.1038/s41419-020-2276-8. Cell Death Dis. 2020. PMID: 32015330 Free PMC article. Review.
-
Promises and pitfalls in erythopoietin-mediated tissue protection: are nonerythropoietic derivatives a way forward?J Investig Med. 2011 Oct;59(7):1073-82. doi: 10.2310/JIM.0b013e3181ed30bf. J Investig Med. 2011. PMID: 20683348 Free PMC article. Review.
-
The therapeutic potential of erythropoiesis-stimulating agents for tissue protection: a tale of two receptors.Blood Purif. 2010;29(2):86-92. doi: 10.1159/000245630. Epub 2010 Jan 8. Blood Purif. 2010. PMID: 20093809 Review.
-
Erythropoietin-mediated tissue protection: reducing collateral damage from the primary injury response.J Intern Med. 2008 Nov;264(5):405-32. doi: 10.1111/j.1365-2796.2008.02024.x. J Intern Med. 2008. PMID: 19017170 Review.
-
Modulation of cellular stress response via the erythropoietin/CD131 heteroreceptor complex in mouse mesenchymal-derived cells.J Mol Med (Berl). 2015 Feb;93(2):199-210. doi: 10.1007/s00109-014-1218-2. Epub 2014 Nov 6. J Mol Med (Berl). 2015. PMID: 25373867 Free PMC article.
Cited by
-
Intranasal Erythropoietin Protects CA1 Hippocampal Cells, Modulated by Specific Time Pattern Molecular Changes After Ischemic Damage in Rats.J Mol Neurosci. 2019 Aug;68(4):590-602. doi: 10.1007/s12031-019-01308-w. Epub 2019 May 3. J Mol Neurosci. 2019. PMID: 31054091
-
Risk of Induction of Corneal Neovascularization with Topical Erythropoietin: An Animal Safety Study.J Ophthalmic Vis Res. 2023 Jul 28;18(3):252-259. doi: 10.18502/jovr.v18i3.13772. eCollection 2023 Jul-Sep. J Ophthalmic Vis Res. 2023. PMID: 38737499 Free PMC article.
-
Bench-to-bedside review: Erythropoietin and its derivatives as therapies in critical care.Crit Care. 2012 Jul 26;16(4):229. doi: 10.1186/cc11315. Crit Care. 2012. PMID: 22839413 Free PMC article. Review.
-
Nonerythropoietic Erythropoietin Mimetic Peptide ARA290 Ameliorates Chronic Stress-Induced Depression-Like Behavior and Inflammation in Mice.Front Pharmacol. 2022 Aug 15;13:896601. doi: 10.3389/fphar.2022.896601. eCollection 2022. Front Pharmacol. 2022. PMID: 36046815 Free PMC article.
-
Erythropoietin Receptor Signaling Supports Retinal Function after Vascular Injury.Am J Pathol. 2020 Mar;190(3):630-641. doi: 10.1016/j.ajpath.2019.11.009. Epub 2020 Feb 21. Am J Pathol. 2020. PMID: 32093902 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

