Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis
- PMID: 22183975
- PMCID: PMC3319524
- DOI: 10.1002/art.34343
Trial of early aggressive therapy in polyarticular juvenile idiopathic arthritis
Abstract
Objective: To determine whether aggressive treatment initiated early in the course of rheumatoid factor (RF)-positive or RF-negative polyarticular juvenile idiopathic arthritis (JIA) can induce clinical inactive disease within 6 months.
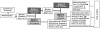
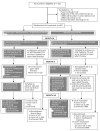
Methods: Between May 2007 and October 2010, a multicenter, prospective, randomized, double-blind, placebo-controlled trial of 2 aggressive treatments was conducted in 85 children ages 2-16 years with polyarticular JIA of <12 months' duration. Patients received either methotrexate (MTX) 0.5 mg/kg/week (maximum 40 mg) subcutaneously, etanercept 0.8 mg/kg/week (maximum 50 mg), and prednisolone 0.5 mg/kg/day (maximum 60 mg) tapered to 0 by 17 weeks (arm 1), or MTX (same dosage as arm 1), etanercept placebo, and prednisolone placebo (arm 2). The primary outcome measure was clinical inactive disease at 6 months. An exploratory phase determined the rate of clinical remission on medication (6 months of continuous clinical inactive disease) at 12 months.
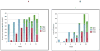
Results: By 6 months, clinical inactive disease had been achieved in 17 (40%) of 42 patients in arm 1 and 10 (23%) of 43 patients in arm 2 (χ(2) = 2.91, P = 0.088). After 12 months, clinical remission on medication was achieved in 9 patients in arm 1 and 3 patients in arm 2 (P = 0.053). There were no significant interarm differences in adverse events.
Conclusion: Although this study did not meet its primary end point, early aggressive therapy in this cohort of children with recent-onset polyarticular JIA resulted in clinical inactive disease by 6 months and clinical remission on medication within 12 months of treatment in substantial proportions of patients in both arms.
Trial registration: ClinicalTrials.gov NCT00443430.
Copyright © 2012 by the American College of Rheumatology.
Figures
References
-
- Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. - PubMed
-
- Gortmaker S. Chronic childhood disorders: prevalence and impact. Ped Clin North Am. 1984;31:3–18. - PubMed
-
- Singsen BH. Rheumatic disease of childhood. Rheum Dis Clin North Am. 1990;16(3):581–99. - PubMed
-
- Lovell DJ, Giannini EH, Reiff A, Cawkwell GD, Silverman ED, Nocton JJ, et al. Etanercept in children with polyarticular juvenile rheumatoid arthritis. Pediatric Rheumatology Collaborative Study Group. N Engl J Med. 2000;342(11):763–9. - PubMed
-
- Lovell DJ, Ruperto N, Goodman S, Reiff A, Jung L, Jarosova K, et al. Adalimumab with or without methotrexate in juvenile rheumatoid arthritis. N Engl J Med. 2008;359(8):810–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- 3R01-AR-049762-04S1/AR/NIAMS NIH HHS/United States
- KL2 RR025749/RR/NCRR NIH HHS/United States
- UL1 RR025764/RR/NCRR NIH HHS/United States
- UL1-RR-025014/RR/NCRR NIH HHS/United States
- R21 AR048355/AR/NIAMS NIH HHS/United States
- 1R21-AR-48355-1/AR/NIAMS NIH HHS/United States
- TL1 RR025748/RR/NCRR NIH HHS/United States
- UL1 RR025014/RR/NCRR NIH HHS/United States
- C06 RR011234/RR/NCRR NIH HHS/United States
- R01 AR049762/AR/NIAMS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- 1R01-AR-049762-01A2/AR/NIAMS NIH HHS/United States
- CO6-RR-11234/CO/NCI NIH HHS/United States
- P60 AR047784/AR/NIAMS NIH HHS/United States
- UL1-RR-024131/RR/NCRR NIH HHS/United States
- P60-AR-047784-07/AR/NIAMS NIH HHS/United States
- UL1 RR025750/RR/NCRR NIH HHS/United States
- TL1-RR-025748/RR/NCRR NIH HHS/United States
- P30-AR-47363/AR/NIAMS NIH HHS/United States
- UL1-RR-025764/RR/NCRR NIH HHS/United States
- UL1-RR-025750/RR/NCRR NIH HHS/United States
- KL2-RR-025749/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

