Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family
- PMID: 22183981
- PMCID: PMC3409716
- DOI: 10.1093/glycob/cwr182
Control of mucin-type O-glycosylation: a classification of the polypeptide GalNAc-transferase gene family
Abstract
Glycosylation of proteins is an essential process in all eukaryotes and a great diversity in types of protein glycosylation exists in animals, plants and microorganisms. Mucin-type O-glycosylation, consisting of glycans attached via O-linked N-acetylgalactosamine (GalNAc) to serine and threonine residues, is one of the most abundant forms of protein glycosylation in animals. Although most protein glycosylation is controlled by one or two genes encoding the enzymes responsible for the initiation of glycosylation, i.e. the step where the first glycan is attached to the relevant amino acid residue in the protein, mucin-type O-glycosylation is controlled by a large family of up to 20 homologous genes encoding UDP-GalNAc:polypeptide GalNAc-transferases (GalNAc-Ts) (EC 2.4.1.41). Therefore, mucin-type O-glycosylation has the greatest potential for differential regulation in cells and tissues. The GalNAc-T family is the largest glycosyltransferase enzyme family covering a single known glycosidic linkage and it is highly conserved throughout animal evolution, although absent in bacteria, yeast and plants. Emerging studies have shown that the large number of genes (GALNTs) in the GalNAc-T family do not provide full functional redundancy and single GalNAc-T genes have been shown to be important in both animals and human. Here, we present an overview of the GalNAc-T gene family in animals and propose a classification of the genes into subfamilies, which appear to be conserved in evolution structurally as well as functionally.
Figures

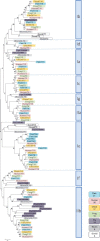


References
-
- Abi-Rached L, Gilles A, Shiina T, Pontarotti P, Inoko H. Evidence of en bloc duplication in vertebrate genomes. Nat Genet. 2002;31:100–105. - PubMed
-
- Almeida R, Levery SB, Mandel U, Kresse H, Schwientek T, Bennett EP, Clausen H. Cloning and expression of a proteoglycan UDP-galactose:β-xylose β1, 4-galactosyltransferase I. A seventh member of the human β4-galactosyltransferase gene family. J Biol Chem. 1999;274:26165–26171. - PubMed
-
- Balakirev ES, Ayala FJ. Pseudogenes: Are they “junk” or functional DNA? Annu Rev Genet. 2003;37:123–151. - PubMed
-
- Bard F, Mazelin L, Pechoux-Longin C, Malhotra V, Jurdic P. Src regulates Golgi structure and KDEL receptor-dependent retrograde transport to the endoplasmic reticulum. J Biol Chem. 2003;278:46601–46606. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

