Sequences prior to conserved catalytic motifs of polysialyltransferase ST8Sia IV are required for substrate recognition
- PMID: 22184126
- PMCID: PMC3307320
- DOI: 10.1074/jbc.M111.322024
Sequences prior to conserved catalytic motifs of polysialyltransferase ST8Sia IV are required for substrate recognition
Abstract
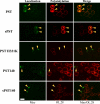
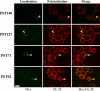
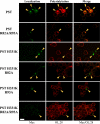
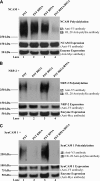
Polysialic acid on the neural cell adhesion molecule (NCAM) modulates cell-cell adhesion and signaling, is required for proper brain development, and plays roles in neuronal regeneration and the growth and invasiveness of tumor cells. Evidence indicates that NCAM polysialylation is highly protein-specific, requiring an initial polysialyltransferase-NCAM protein-protein interaction. Previous work suggested that a polybasic region located prior to the conserved polysialyltransferase catalytic motifs may be involved in NCAM recognition, but not overall enzyme activity (Foley, D. A., Swartzentruber, K. G., and Colley, K. J. (2009) J. Biol. Chem. 284, 15505-15516). Here, we employ a competition assay to evaluate the role of this region in substrate recognition. We find that truncated, catalytically inactive ST8SiaIV/PST proteins that include the polybasic region, but not those that lack this region, compete with endogenous ST8SiaIV/PST and reduce NCAM polysialylation in SW2 small cell lung carcinoma cells. Replacing two polybasic region residues, Arg(82) and Arg(93), eliminates the ability of a full-length, catalytically inactive enzyme (PST H331K) to compete with SW2 cell ST8SiaIV/PST and block NCAM polysialylation. Replacing these residues singly or together in ST8SiaIV/PST substantially reduces or eliminates NCAM polysialylation, respectively. In contrast, replacing Arg(82), but not Arg(93), substantially reduces the ability of ST8SiaIV/PST to polysialylate neuropilin-2 and SynCAM 1, suggesting that Arg(82) plays a general role in substrate recognition, whereas Arg(93) specifically functions in NCAM recognition. Taken together, our results indicate that the ST8SiaIV/PST polybasic region plays a critical role in substrate recognition and suggest that different combinations of basic residues may mediate the recognition of distinct substrates.
Figures



References
-
- Kojima N., Yoshida Y., Kurosawa N., Lee Y. C., Tsuji S. (1995) Enzymatic activity of a developmentally regulated member of the sialyltransferase family (STX). Evidence for α2,8-sialyltransferase activity toward N-linked oligosaccharides. FEBS Lett. 360, 1–4 - PubMed
-
- Eckhardt M., Mühlenhoff M., Bethe A., Koopman J., Frosch M., Gerardy-Schahn R. (1995) Molecular characterization of eukaryotic polysialyltransferase-1. Nature 373, 715–718 - PubMed
-
- Scheidegger E. P., Sternberg L. R., Roth J., Lowe J. B. (1995) A human STX cDNA confers polysialic acid expression in mammalian cells. J. Biol. Chem. 270, 22685–22688 - PubMed
-
- Angata K., Fukuda M. (2003) Polysialyltransferases. Major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie 85, 195–206 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

