Chromosomal instability substantiates poor prognosis in patients with diffuse large B-cell lymphoma
- PMID: 22184286
- PMCID: PMC3244806
- DOI: 10.1158/1078-0432.CCR-11-2049
Chromosomal instability substantiates poor prognosis in patients with diffuse large B-cell lymphoma
Abstract
Purpose: The specific role of chromosomal instability (CIN) in tumorigenesis has been a matter of conjecture. In part, this is due to the challenge of directly observing chromosome mis-segregation events as well as the inability to distinguish the role of CIN, which consists of increased rates of chromosome mis-segregation, from that of aneuploidy, which is a state of nondiploid chromosome number.
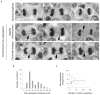
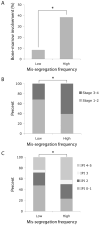
Experimental design: Here, we examine the contribution of CIN to the prognosis of patients diagnosed with diffuse large B-cell lymphoma (DLBCL) by directly surveying tumor cells, fixed while undergoing anaphase, for evidence of chromosome mis-segregation. Hematoxylin and eosin-stained samples from a cohort of 54 patients were used to examine the relationship between frequencies of chromosome mis-segregation and patient prognosis, overall survival, and response to treatment.
Results: We show that a two-fold increase in the frequency of chromosome mis-segregation led to a 24% decrease in overall survival and 48% decrease in relapse-free survival after treatment. The HR of death in patients with increased chromosome mis-segregation was 2.31 and these patients were more likely to present with higher tumor stage, exhibit tumor bone marrow involvement, and receive a higher International Prognostic Index score.
Conclusions: Increased rates of chromosome mis-segregation in DLBCL substantiate inferior outcome and poor prognosis. This is likely due to increased heterogeneity of tumor cells leading to a larger predilection for adaptation in response to external pressures such as metastasis and drug treatments. We propose that targeting CIN would yield superior prognosis and improved response to chemotherapeutic drugs.
©2011 AACR.
Conflict of interest statement
Figures


References
-
- Cimini D. Merotelic kinetochore orientation, aneuploidy, and cancer. Biochim Biophys Acta. 2008;1786:32–40. - PubMed
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386:623–7. - PubMed
-
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instabilities in human cancers. Nature. 1998;396:643–9. - PubMed
-
- Lingle WL, Lukasiewicz K, Salisbury JL. Deregulation of the centrosome cycle and the origin of chromosomal instability in cancer. Genome Instability in Cancer Development. 2005;570:393–421. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

