Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease
- PMID: 22184378
- PMCID: PMC4979082
- DOI: 10.1200/JCO.2011.34.7898
Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease
Abstract
Purpose: BCL2 overexpression is a hallmark of chronic lymphocytic leukemia (CLL). The novel BH3 mimetic navitoclax (ABT-263) specifically inhibits BCL2 and related proteins BCL-x(l) and BCL-w, potently inducing apoptosis of CLL cells in vitro. A phase I trial in patients with CLL was conducted to evaluate the safety, pharmacokinetics, and biologic activity of oral navitoclax.
Patients and methods: Twenty-nine patients with relapsed or refractory CLL received daily navitoclax for 14 days (10, 110, 200, or 250 mg/d; n = 15) or 21 days (125, 200, 250, or 300 mg/d; n = 14) of each 21-day cycle. Dose escalation decisions were informed by continual reassessment methodology.
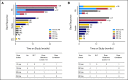
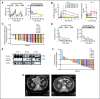
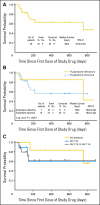
Results: Lymphocytosis was reduced by more than 50% in 19 of 21 patients with baseline lymphocytosis. Among 26 patients treated with navitoclax ≥ 110 mg/d, nine (35%) achieved a partial response and seven maintained stable disease for more than 6 months. Median treatment duration was 7 months (range, 1 to ≥ 29 months). Median progression-free survival was 25 months. Activity was observed in patients with fludarabine-refractory disease, bulky adenopathy, and del(17p) CLL. Thrombocytopenia due to BCL-x(l) inhibition was the major dose-limiting toxicity and was dose-related. Low MCL1 expression and high BIM:MCL1 or BIM:BCL2 ratios in leukemic cells correlated with response. We determined that the navitoclax dose of 250 mg/d in a continuous dosing schedule was optimal for phase II studies.
Conclusion: BCL2 is a valid therapeutic target in CLL, and its inhibition by navitoclax warrants further evaluation as monotherapy and in combination in this disease.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures

Comment in
-
Targeted therapy for chronic lymphocytic leukemia: a glimpse into the future.J Clin Oncol. 2012 Feb 10;30(5):469-70. doi: 10.1200/JCO.2011.37.8612. Epub 2011 Dec 19. J Clin Oncol. 2012. PMID: 22184387 No abstract available.
-
Venetoclax Adds a New Arrow Targeting Relapsed CLL to the Quiver.Cancer Cell. 2016 Jan 11;29(1):3-4. doi: 10.1016/j.ccell.2015.12.010. Cancer Cell. 2016. PMID: 26766586
References
-
- O'Brien SM, Cunningham CC, Golenkov AK, et al. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:7697–7702. - PubMed
-
- Oltersdorf T, Elmore SW, Shoemaker AR, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. - PubMed
-
- Tse C, Shoemaker AR, Adickes J, et al. ABT-263: A potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

